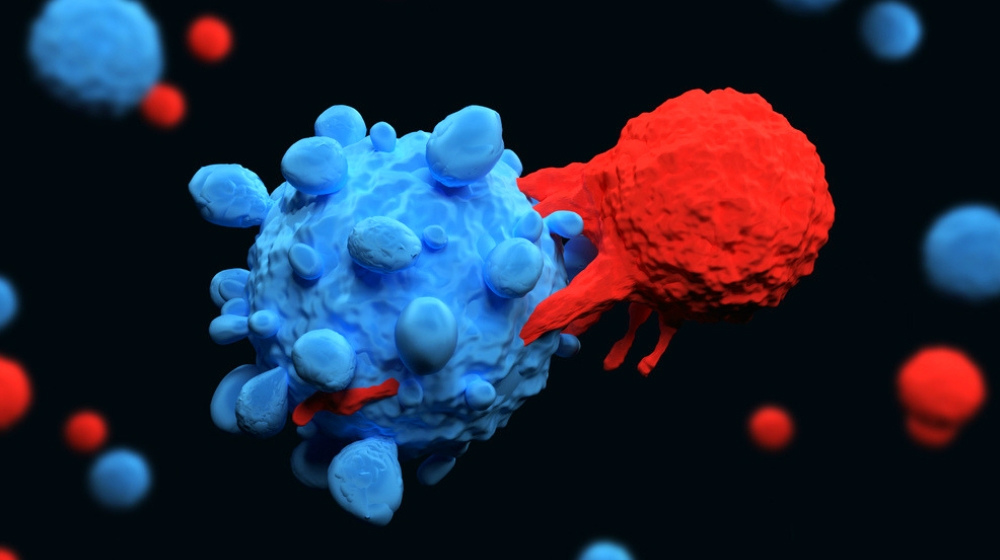
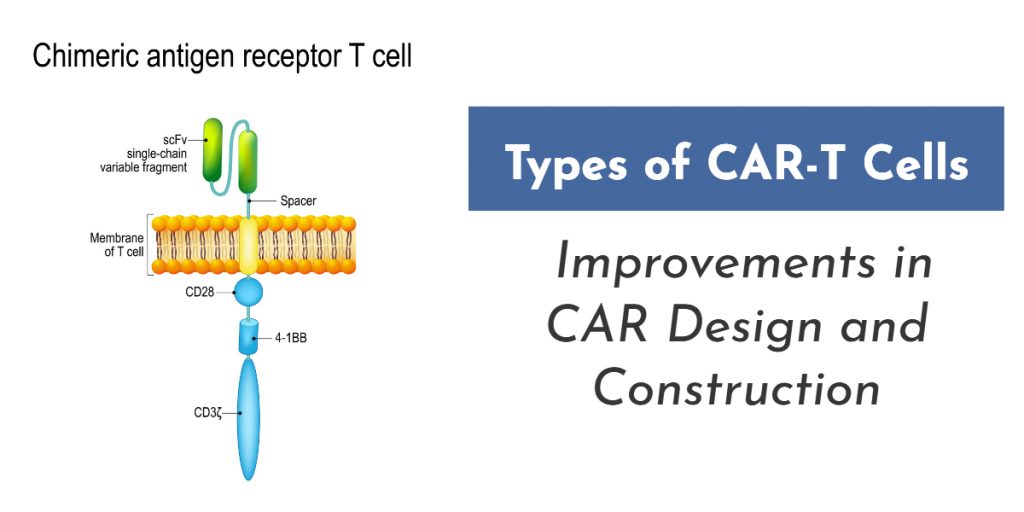
Can T cells be an effective approach to cancer treatment? CAR T-cell therapy, as this cutting-edge treatment is called, uses a patient’s own immune system to help fight cancers in the body. The result is a natural treatment that proves effective against a variety of blood cancers and may have hope for other cancers in the future as well.
T-cells are the workhorses of our immune system and they play a key role in directing the immune response and killing cells infected by pathogens.
Using T Cells For Cancer Treatment
In this article:
- CAR-T Cell Therapy Treatments for Treatment of Cancer
- What Causes Cancer?
- How Does the Immune System Work – and What Are Its Limits?
- What Is Immunotherapy?
- Are There Different Types of Immunotherapy?
- What Are T Cells?
- What Is CAR-T Cell Therapy?
- How Does CAR-T Cell Therapy Work?
- Who Is CAR-T Cell Therapy Right For?
- Why Doesn’t CAR-T Cell Therapy Work for Solid Tumors?
- Are There Any Risks?
- What Are the Benefits of CAR-T?
- How Effective Is CAR-T Treatment?
- Progress in CAR-T Cell Therapy Development