The global CAR-T market has grown exponentially since the first CAR-T approval reached the market in 2017. Over the past four years, a growing pipeline of CAR-T clinical trials have been accumulating, leading to six global approvals and an explosion of CAR-T market competitors worldwide. [Read more…]
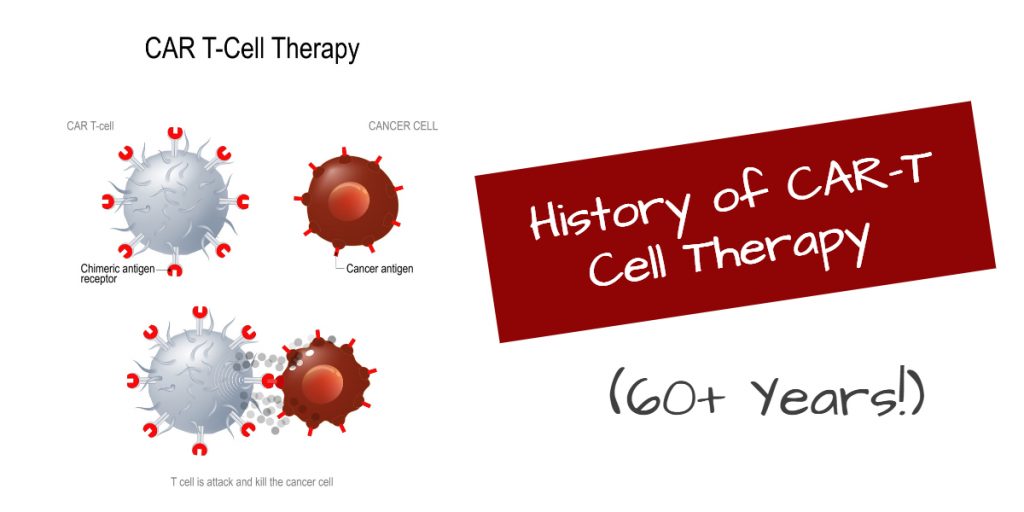
History of CAR-T Cell Therapy Spans 60+ Years
The groundbreaking approvals of two CAR-T therapies in 2017 was the climax of more than sixty years of devoted research. In the 1950s, the discovery of bone marrow transplantation laid the foundation for developing CAR-T therapy, as it was the first time that living cells were infused into blood cancer patients for the control of cancer.
As early as 1900s, researchers were becoming aware that T cells could easily search, recognize and kill cancer cells. To do this, the cells need a “guide” to direct them to the tumors, and thus, antibodies emerged as a valuable scientific and medical tool. [Read more…]
What is CAR-T Cell Therapy? A New Way to Treat Cancer
CAR-T cell therapy is as a type of immunotherapy that teaches T cells to recognize and destroy cancer. CAR-T cell therapy has demonstrated promising results in a range of patients across the globe. In some patients, this can lead to the total elimination of the cancer. In others, there is a significant improvement of the disease.
For those who are facing cancer, it is important to answer the question “What is CAR-T?” This guide will answer the most common questions about CAR-T cell therapy for readers who want to understand this novel technology platform for treating cancer. [Read more…]
Legend Biotech’s CARVYKTI™ Becomes Latest CAR-T Therapy Approved by U.S. FDA
-
CARVYKTI™ (ciltacabtagene autoleucel), BCMA-Directed CAR-T Therapy, Receives U.S. FDA Approval for the Treatment of Adult Patients With Relapsed or Refractory Multiple Myeloma
-
CARVYKTI™ marks the first product approved by a health authority for Legend Biotech
-
Approval is primarily based on the pivotal phase 1b/2 CARTITUDE-1 study, which demonstrated an overall response rate (ORR) of 98 percent in patients with refractory or relapsed multiple myeloma after four or more prior lines of therapy including proteasome inhibitor, immunomodulatory agent and anti-CD38 monoclonal antibody1
New Frontiers in Gene-Editing and Stem Cell Innovation – Interview with Dr. Chen-Tsai of Applied StemCell
 I had the honor of interviewing Dr. Ruby Yanru Chen-Tsai, Chief Scientific Officer and Co-founder of Applied StemCell Inc. (ASC), a fast growing biotechnology company in Milpitas, California. Specializing in gene-editing technologies and stem cell innovation, ASC offers an optimized series of tools for basic research study, drug discovery, bio-processing, bio-production and preclinical applications.
I had the honor of interviewing Dr. Ruby Yanru Chen-Tsai, Chief Scientific Officer and Co-founder of Applied StemCell Inc. (ASC), a fast growing biotechnology company in Milpitas, California. Specializing in gene-editing technologies and stem cell innovation, ASC offers an optimized series of tools for basic research study, drug discovery, bio-processing, bio-production and preclinical applications.
In this fascinating interview, we discuss Dr. Yanru Chen-Tsai’s impressive background, ASC’s unique product portfolio, its TARGATT™ gene editing technology, and the company’s future trajectory. [Read more…]
- « Previous Page
- 1
- 2
- 3
- 4
- 5
- …
- 12
- Next Page »