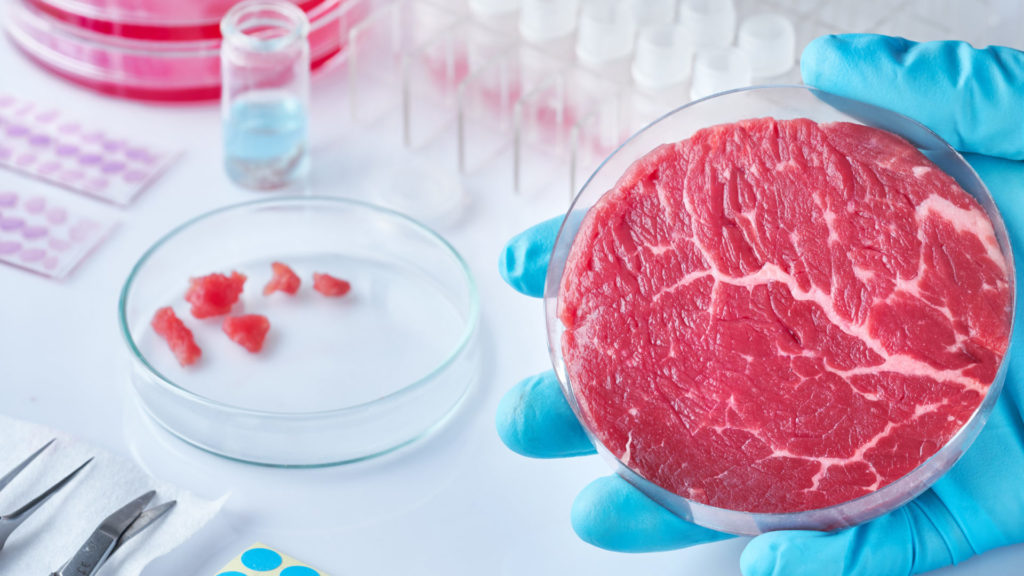
Lab-grown meat is fast becoming a real alternative to its farm-grown counterpart as billionaire entrepreneurs and industry heavyweights invest into start-ups from across the nascent field of “cellular agriculture”. Produced via stem cells derived from cows, pigs, fish, sheep, or other livestock, the emerging clean meat industry has the potential to transform the global food market and create a new trillion dollar industry in the process. [Read more…]
What Stem Cell Clinics Do You Trust?
On a global basis, stem cell clinics have proliferated in recent years. This article explores leading stem cell centers within the U.S. and worldwide. Read on to learn about this emerging area of medicine. [Read more…]
How to Find a Stem Cell Clinical Trial for Your Condition
Are you or a loved on suffering from arthritis, multiple sclerosis (MS), COPD, Alzheimer’s disease, asthma, or other chronic condition? If so, how do you find a stem cell clinical trial?
This article provides the answers, including links to find a clinical trials near you, as well as links to clinical trials for specific conditions.
Cultivated Meat Market: Lab-Grown Meat Grows by Leaps and Bounds
Cultivated meat refers to meat created using cell culture techniques within a laboratory or manufacturing facility. It is produced by growing master cells collected from cattle, chicken, pigs, fish, lamb, and other livestock. Cultivated meat is ethically produced, because livestock is not used within the manufacturing processes beyond collecting the initial cells required for cell culture. [Read more…]
What is Lab-Grown Meat and Why Does It Matter?
The potential of lab-grown meat has captured the imagination of investors, researchers, and consumers alike. In the late 1990s, Willem van Eelen was the first applicant to file the first patent for a cultured meat manufacturing method. Although the lab-grown meat industry has only existed for about 25 years, 2020 was a turning point because a cultured chicken product developed by the company “Eat Just” made its debut on a restaurant menu in Singapore. [Read more…]
- 1
- 2
- 3
- …
- 10
- Next Page »