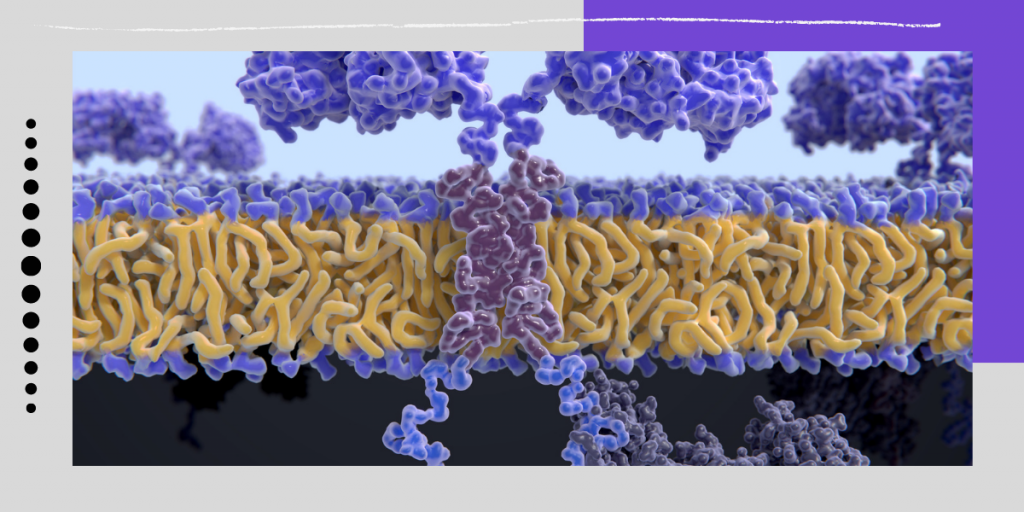
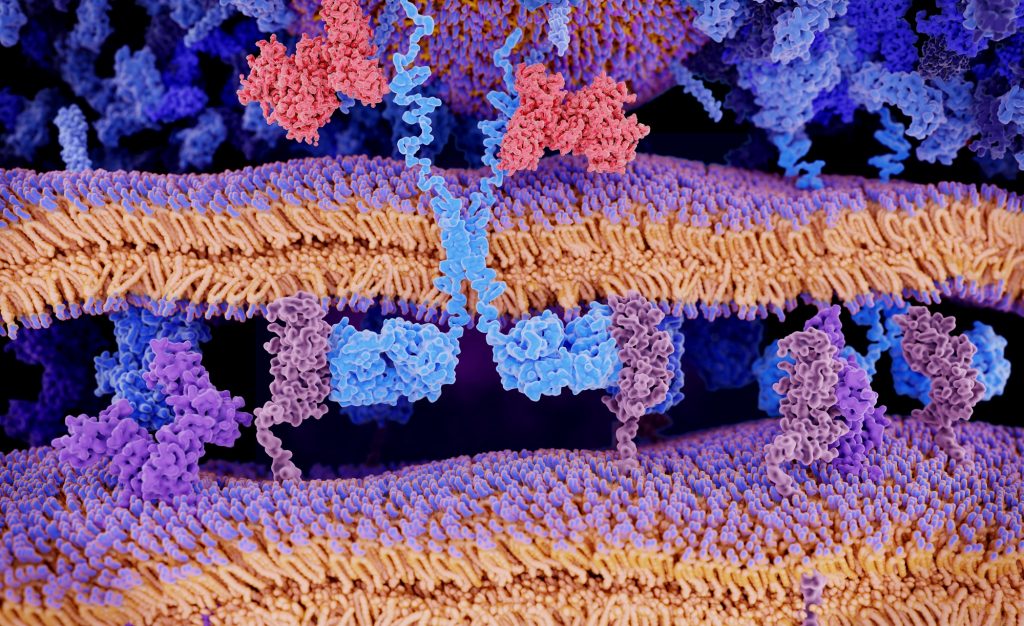
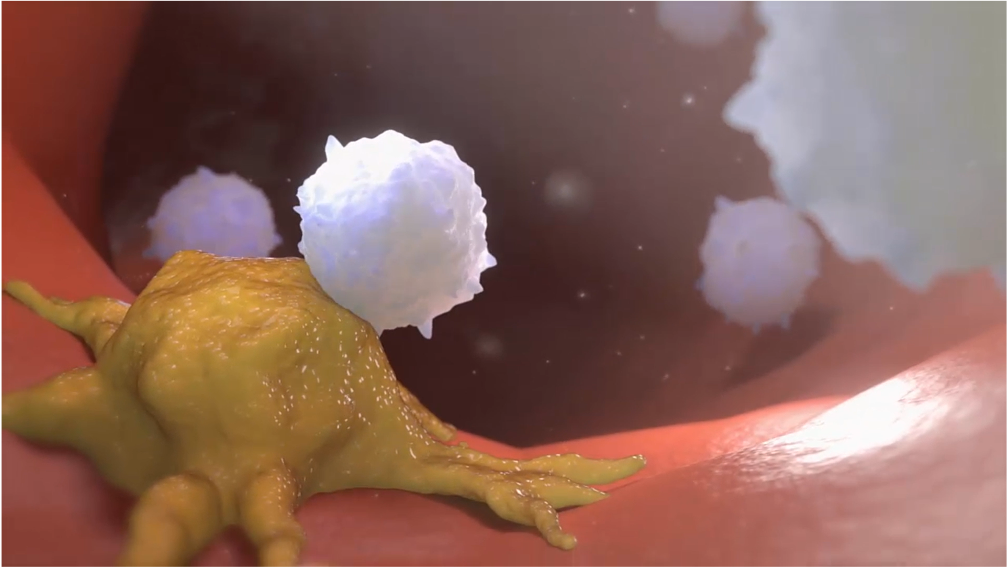
CAR-T is a promising type of immunotherapy where doctors collect immune cells, modify them in a laboratory, and provide them the capacity to recognize and kill cancer cells. When infused into a patient, the cells get multiplied and stay in the body as “living drugs.”
Since the approval of the first CAR-T cell therapeutic in 2017, widespread research, proliferating clinical trials, aggressive M&A activity, and lucrative IPOs have created a robust CAR-T cell market. [Read more…]