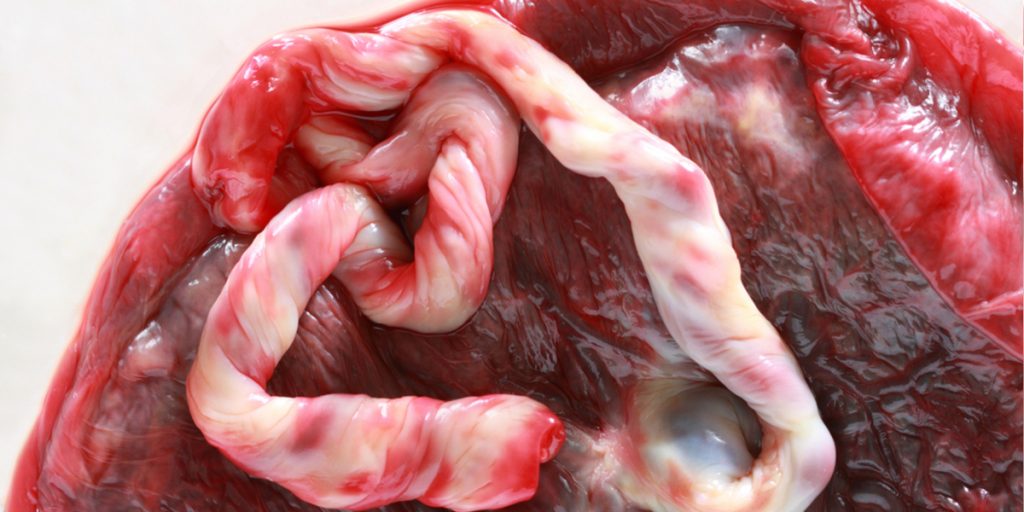
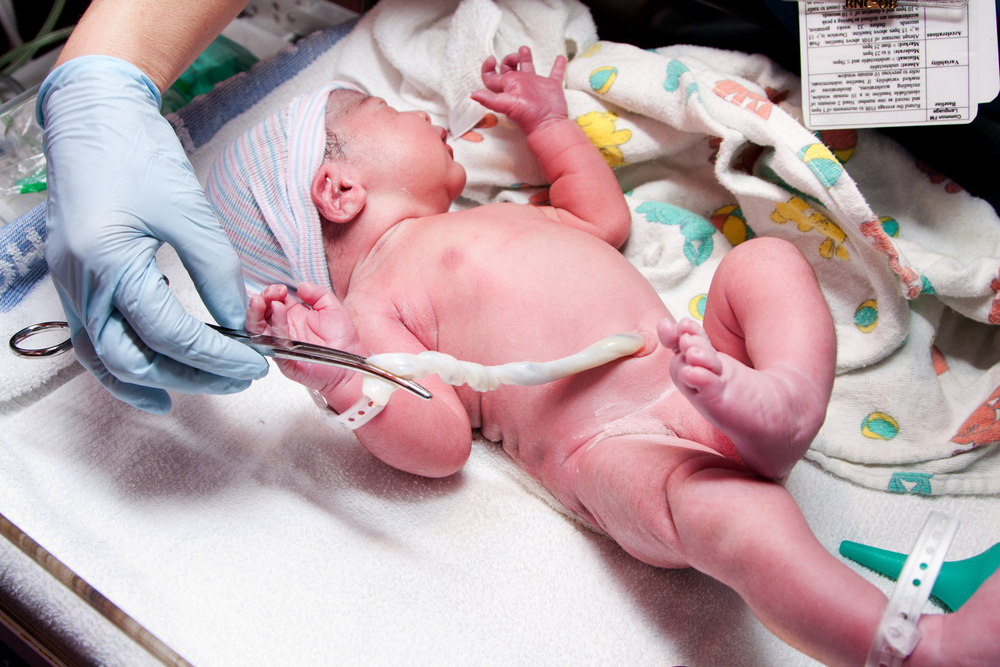
What are placental stem cells and what are placental stem cells used for? As placental stem cells get explored for a variety of therapeutic applications, these questions become increasingly important. The opportunity to collect placental stem cells is also a once in a lifetime opportunity. [Read more…]
Celularity – Big Bucks, Billionaire Investors, Bold Dreams
Celularity is a biotechnology company that specializes in leveraging biologically active cell populations within the postpartum human placenta. Founded on the pioneering work of Robert Hariri, MD, PhD, in human placenta-derived cellular therapeutics and biomaterials, Celularity is uniquely positioned to harness the potential of most biocreative event on earth: human birth. [Read more…]
Placental-Derived Allogeneic Stem Cells Meet the Needs of the Market and the Patient
As an increasing number of stem cell therapies advance through clinical trials, the healthcare industry’s focus comes to looking at which cells, allogeneic or autologous, are more likely to be commercially feasible. Proving safety and efficacy for a specific indication is a large enough challenge for any treatment, but for stem cell therapies in particular, commercial viability may be just as big a hurdle.
Israel-based Pluristem Therapeutics (NASDAQ:PSTI), may be the only stem cell company that can mass produce immune-privileged allogeneic cells at a scale and price that not only makes them commercially viable, but moreover makes stem cell therapy a preferred method of treatment for patients and payors alike. A proprietary 3D stem cell expansion technology which uses placental-derived cells is the key. [Read more…]
How the Cord Blood and Tissue Market is Changing
The global cord blood and tissue banking market is consolidating, driven by the emergence of holding companies and aggressive M&A activity. This is creating risks for cord blood market participants, as well as new opportunities.
Serious threats to the industry include:
- Low rates of stored cord blood utilization
- Utilization of bone marrow and peripheral blood stem cells as alternative sources of stem cells for HSCT
- Growing prevalence of haploidentical transplantation
- Expensive transplant procedures, ranging from $200-300K
- Difficulty educating Obstetricians about the future value of cellular therapies
- Low rates of market penetration
- Low rates of cord blood awareness among expectant parents
Pluristem Therapeutics, Interview with Yaky Yanay, President and Co-CEO
 Pluristem Therapeutics (PSTI) is a clinical-stage company that is a proprietary 3D manufacturing technology to develop placental cell therapies for conditions that include ischemia, muscle injury, and exposure to radiation. I had the honor of interviewing Mr. Yaky Yanay, President and Co-CEO of Pluristem Therapeutics.
Pluristem Therapeutics (PSTI) is a clinical-stage company that is a proprietary 3D manufacturing technology to develop placental cell therapies for conditions that include ischemia, muscle injury, and exposure to radiation. I had the honor of interviewing Mr. Yaky Yanay, President and Co-CEO of Pluristem Therapeutics.
In this interview, we discuss the history of Pluristem Therapeutics, its clinical-stage products, intellectual property (IP) portfolio, marketing strategy and future directions.
Enjoy these insights into Pluristem Therapeutics, a world-class leader in cell therapy manufacturing and placental-derived products. [Read more…]