How much is stem cell therapy? According to analysis by BioInformant, the cost of stem cell therapy ranges from less than $5,000 for simple procedures to $25,000 or more for complex ones. In general, stem cell treatment procedures are paid out-of-pocket by patients, because they are not covered by medical insurance. The cost of platelet rich therapy (PRP), which can be used separately or in conjunction with stem cell therapy, is typically $500-700, but may be as high as $2,000 at some locations. [Read more…]
How Long Does It Take for Stem Cell Therapy to Work?
Are you considering having stem cell therapy? If so, you’re probably wondering, how long does it take for stem cell therapy to work?
In this article, we will go over what stem cell therapy is, how the various procedures work, and what to expect post stem cell treatment. It’s important to know that extremely few stem cell procedures are FDA approved, so you will need to do your due diligence.
Keep reading to get informed on this topic. [Read more…]
4 Major Areas of Commercialization for iPS Cells
Since the discovery of induced pluripotent stem cells in 2006, a great deal of basic research has been done to understand how to produce, manipulate, and utilize the stem cell type. In addition to this important basic research, a great deal of applied (“translational”) results has been done with the cell type. Induced pluripotent stem cells (also called iPS cells or iPSCs) are revolutionizing regenerative medicine because they represent a potential route for producing patient-specific stem cells for research or clinical use.
In the future, iPS cells will facilitate progress in personalized medicine by allowing a patient to use his or her own cells. In addition, iPSCs also show great promise in other areas, such as phamaco-toxicological screening, by allowing disease modeling and safety assessment of potential new drugs under development, in short, facilitating the study of a “disease in a dish.”
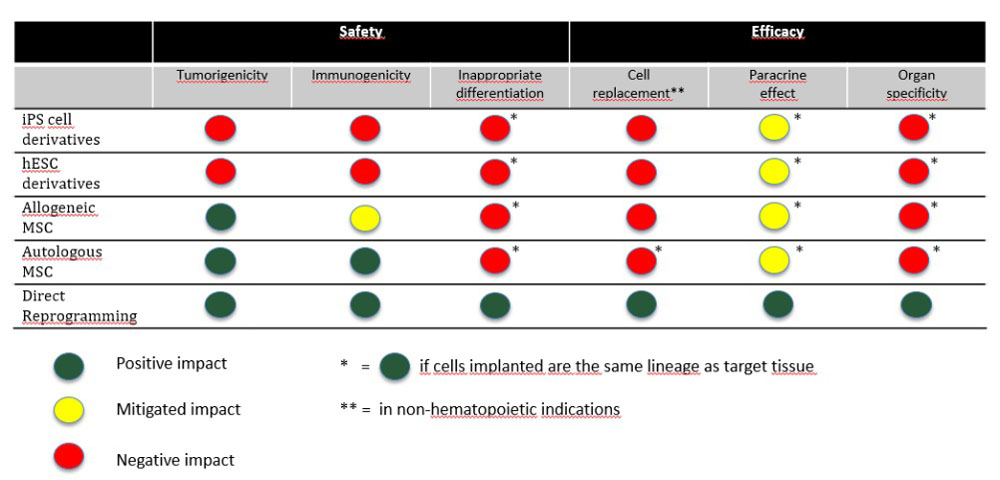
The Future of Stem Cell Therapeutics – Balancing Safety and Efficacy
What do we know of the safety and efficacy of stem cell therapeutics? Over 50 years have passed since the discovery of hematopoietic stem cells (bone marrow transplantation to cure diseases such as leukemia), over 20 years since the discovery of human embryonic stem cells (hESC), and 15 years since the discovery of induced pluripotent stem cells (iPS cells). There are now thousands of stem cell trials underway in clinics worldwide.
Despite this experience, what do we actually know about the safety and efficacy of stem cell therapeutics? [Read more…]
Can New Manufacturing Platform Reduce “Sky High” Costs of Stem Cell Therapies?
The LA Times released a compelling article highlighting the mounting evidence that stem cell treatments will be some of the highest priced treatments within the medical marketplace. Titled “Sky-high price of new stem cell therapies is a growing concern,” the author Michael Hiltzik explores the often exorbitant costs associated with stem cell procedures.
In a powerful statement that summarizes the author’s position, he writes, “The evidence is already mounting that stem cell and other advanced biologic treatments will be among the most expensive therapies in the medical arsenal.” As examples of expensive cell therapy procedures, Hiltzik cites that Prochymal, a mesenchymal stem cell treatment approved in Canada, can costs as much as $200K, while Provenge, a cell-based vaccine for prostate cancer, can cost nearly $100K to extend a patient’s life by a few months. [Read more…]