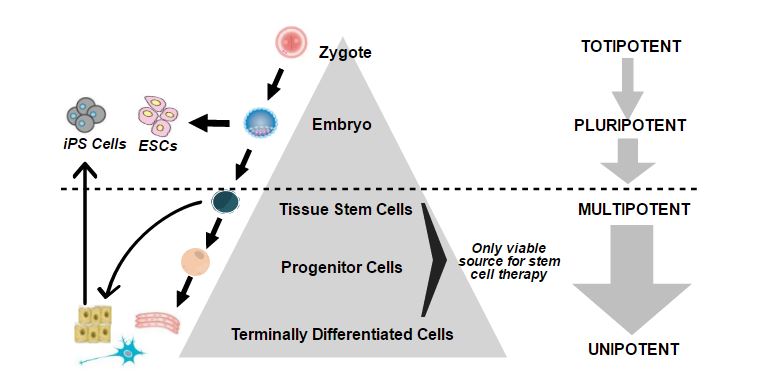
Direct cell reprogramming is the in vitro (laboratory) or in vivo (in the body) reprogramming of somatic cells into other cell types without the need for an intermediate pluripotent state. Another term for direct cell reprogramming is transdifferentiation, which is the direct conversion of one differentiated cell type into another.
There has been tremendous activity over the last decade in the development of stem cells therapeutics, which have relied on stem cell differentiation protocols, as well as techniques for direct cell reprogramming. Today, there are over 7,149+ stem cell trials registered on ClinicalTrials.gov and stem cell publications have increased dramatically in recent years, recently surpassing 500,000+ on PubMed.gov.
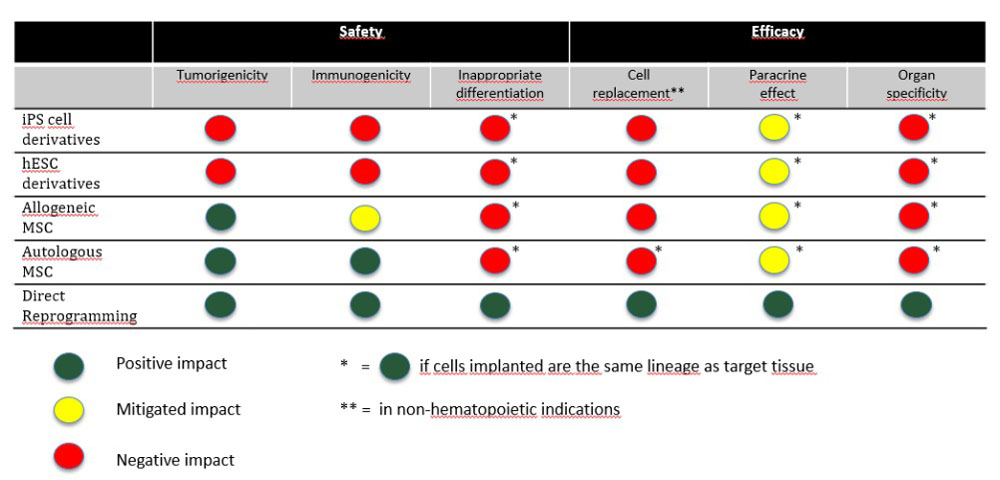
However, for all their promise, stem cells are yet to deliver on this therapeutic potential, and this failure appears due to various technical challenges that still need to be overcome. [Read more…]