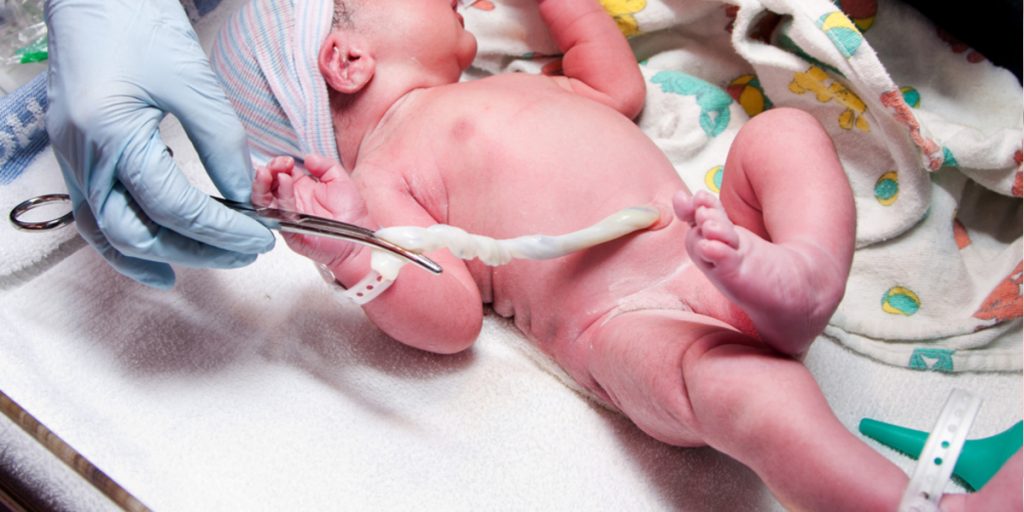
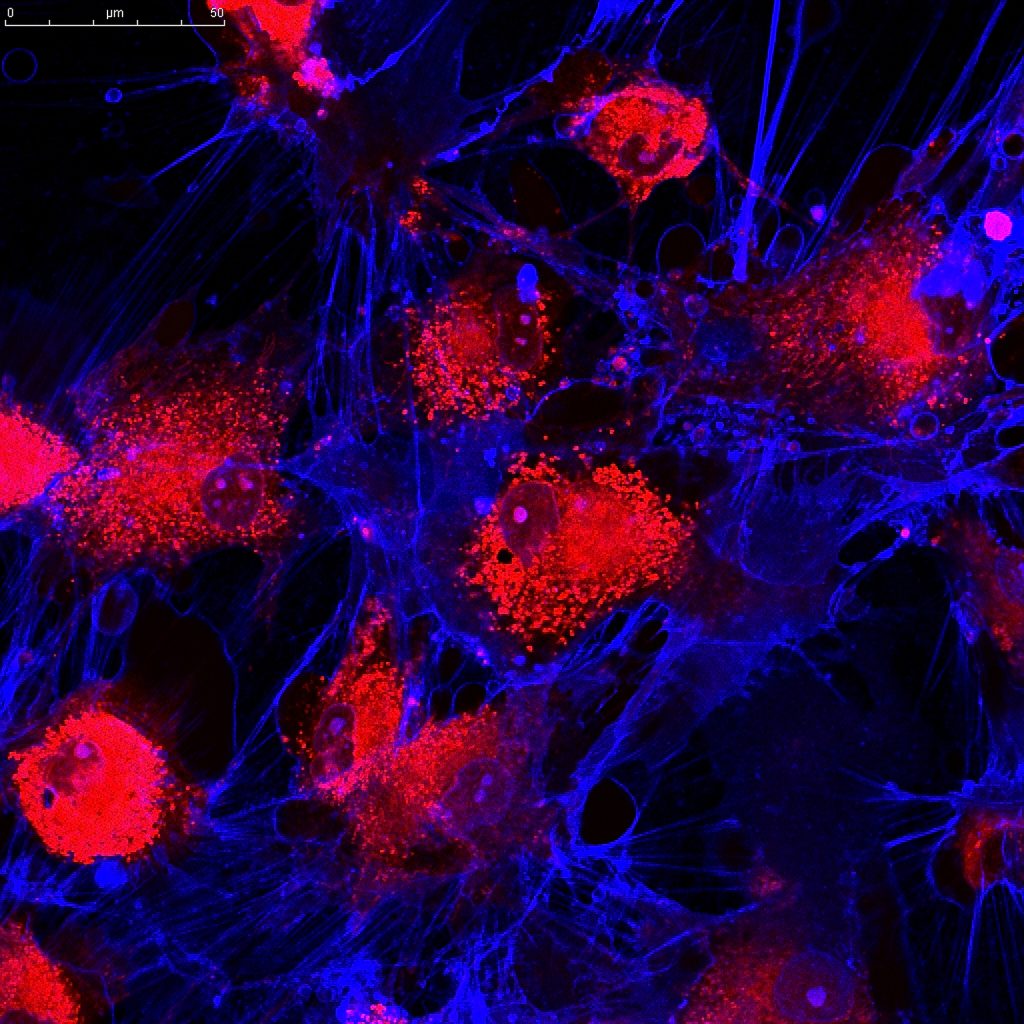
Cord tissue banking is the processing and storage of a newborn’s umbilical cord tissue or the components contains, such as Wharton’s jelly. Wharton’s jelly is a thick, gelatinous substance present within the human umbilical cord that contains a range of important cell types, such as mesenchymal stem cells (MSCs), fibroblasts and macrophages.
In this article: