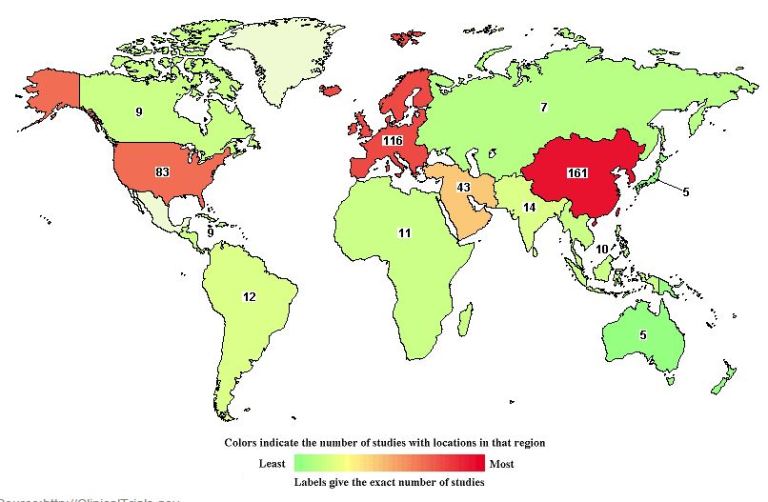
Are you or a loved on suffering from arthritis, multiple sclerosis (MS), COPD, Alzheimer’s disease, asthma, or other chronic condition? If so, how do you find a stem cell clinical trial?
This article provides the answers, including links to find a clinical trials near you, as well as links to clinical trials for specific conditions.