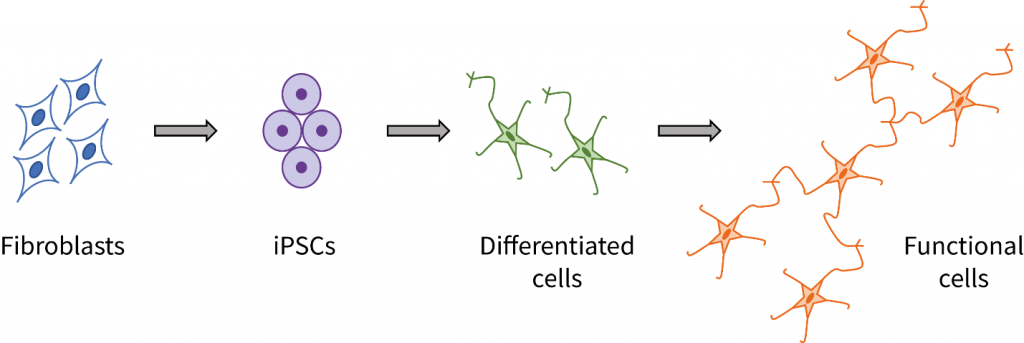
Despite progress involving the use of induced pluripotent stem cells (iPSCs) within disease modeling and drug discovery applications, it will be a long path to achieve the broad-scale use of iPSC-derived cell types in human patients. [Read more…]
Induced Pluripotent Stem Cell (iPS Cell) Applications in 2024
Since the discovery of induced pluripotent stem cells (iPSCs) in 2006, a large and thriving research products market has emerged, largely because the cells are non-controversial and can be generated directly from adult cells. It is clear that iPSCs represent a lucrative market segment, because methods for commercializing this cell type are expanding every year and clinical studies investigating iPSCs are swelling in number. [Read more…]
Clean Meat Market: Stem Cell Derived “Clean Meat” Attracts Billionaires
Lab-grown meat is fast becoming a real alternative to its farm-grown counterpart as billionaire entrepreneurs and industry heavyweights invest into start-ups from across the nascent field of “cellular agriculture”. Produced via stem cells derived from cows, pigs, fish, sheep, or other livestock, the emerging clean meat industry has the potential to transform the global food market and create a new trillion dollar industry in the process. [Read more…]
What Are iPSCs, Who is Funding Them, and What Trials are Underway?
Induced pluripotent stem cells (iPS cells) can be made by reprogramming mature adult cells back into an embryonic-like state. Derived from skin or blood cells, iPS cells are not controversial, because they are made from adult cells. As pluripotent stem cells, they can give rise to all of the tissues that form the human body. [Read more…]
Development of iPSC-derived Disease Models
Human induced pluripotent stem cell (iPSC)-based models are a valuable resource for studying disease mechanisms in vitro at the cellular level[1], screening potential new therapeutics[2], and investigating the propensity and mechanism for the development of toxic side effects caused by a drug treatment[3]. Such iPSC-based models enable research to be performed under defined experimental conditions and in a reproducible manner. [Read more…]
- 1
- 2
- 3
- …
- 8
- Next Page »