 Oscine Therapeutics is a newcomer to the stem cell sector, a private biotech company exploring mechanisms for treating neurodegenerative disorders through the use of replacing lost or damaged glia cells. The company is based the renowned research of Steve Goldman, M.D., Ph.D., co-director of the University of Rochester Medical Center (URMC) Center for Translational Neuromedicine.
Oscine Therapeutics is a newcomer to the stem cell sector, a private biotech company exploring mechanisms for treating neurodegenerative disorders through the use of replacing lost or damaged glia cells. The company is based the renowned research of Steve Goldman, M.D., Ph.D., co-director of the University of Rochester Medical Center (URMC) Center for Translational Neuromedicine.
The focus of Dr. Goldman’s research has been to manipulate the chemical signaling of pluripotent stem cell types, including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPS cells), to create healthy glia cells. [Read more…]
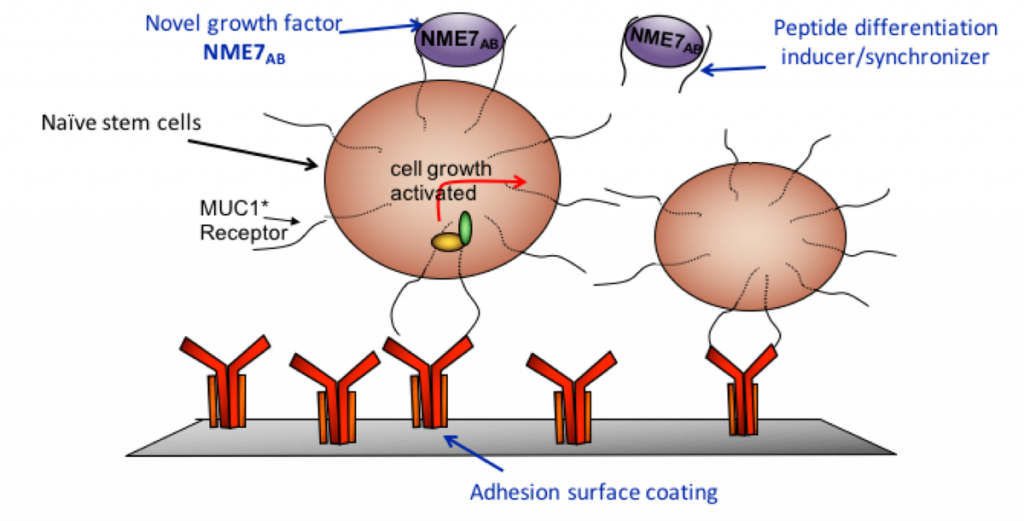




 On July 2nd, 2018,
On July 2nd, 2018, 

















