Since the start of 2016, there has been a lot of licensing activity surrounding stem cell technologies, with key agreements linked between the Coriell Institute for Medical Research and Drs. Jeanne Loring and Franz Josef Muller (access to a bioinformatics assay that can assess the pluripotency of human induced pluripotent stem cells), Avapecia Life Sciences Corporation and Oceans Five-O Development Corporation (use of a cell insolation and enrichment technology to identify stem cell candidates for cell therapy applications), and most recently, TheraKine and Cell Care Therapeutics (use of injectable sustained release technology within stem cell therapies for eye disease). [Read more…]
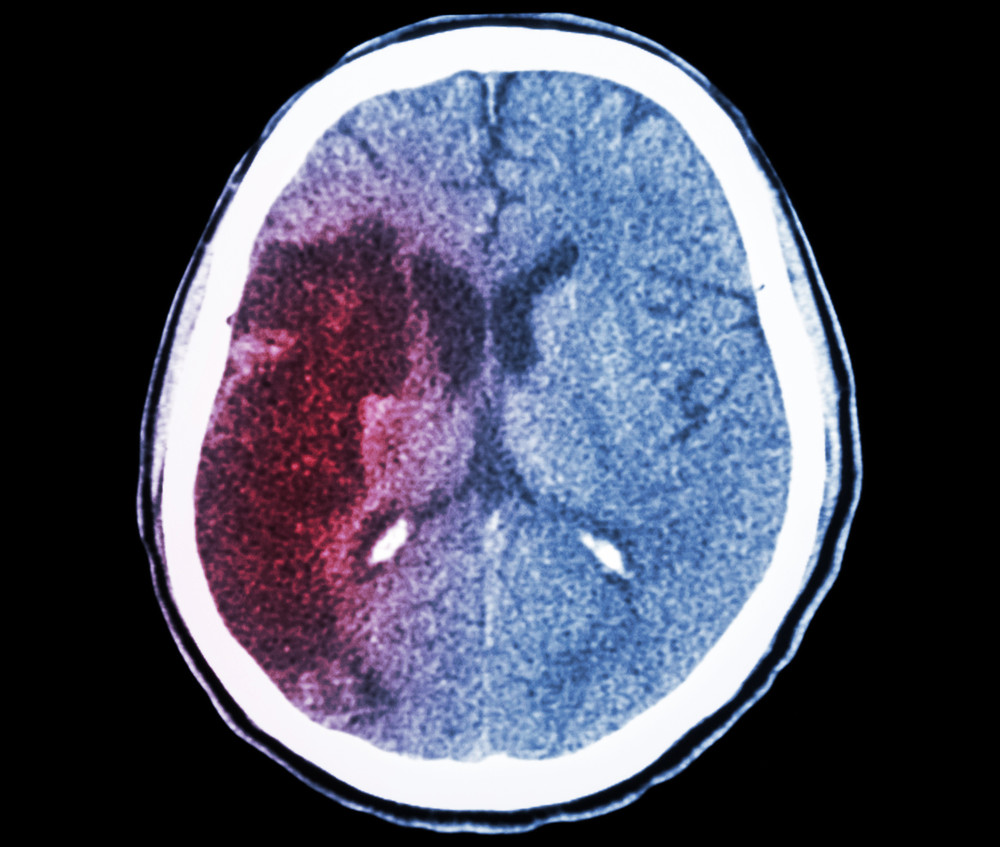
Hemostemix Poised to Expand Phase-2 Clinical Trial of Lead Product ACP-01 to the United States
December 22, 2015; Toronto, Ontario – Hemostemix Inc. (“Hemostemix” or the “Company”) (TSXV: HEM), a clinical-stage autologous cell-therapy company, announced today the Company’s progress in expanding the international, multicenter, phase-2 clinical trial of its lead product ACP-01 for critical limb ischemia (CLI) to the United States.
The ongoing phase-2 clinical trial investigates the safety and efficacy of ACP-01, which uses angiogenic progenitor cells to combat the life-threatening complications of CLI. These proprietary cells are grown from a patient’s own blood and, once injected into his or her diseased tissue, are able to support the formation of new blood vessels. The clinical trial currently recruits patient at two sites in Canada and four sites in South Africa.
“After receiving clearance from the FDA to recruit patients in the United States, we’ve been engaged in a multistep site-initiation process with qualified medical centers that involves executing nondisclosure agreements and discussing our clinical trial protocol,” said Dr. Hardean E. Achneck, vice president of clinical research and operations at Hemostemix. “I’m encouraged and inspired by the positive feedback on the design of our clinical trial that we’ve been receiving from vascular surgeons at such leading U.S. medical centers as Houston Methodist DeBakey Heart & Vascular Center, University of California Davis Vascular Center, Ronald Reagan UCLA Medical Center, Temple University, Malcom Randall Veterans Affairs Medical Center, and Yale University. We are discussing the terms of contractual agreements with some of these sites, and several sites are currently in the process of submitting study documents to their respective institutional review boards.”
“Leading medical centers in the United States are increasingly recognizing the groundbreaking potential of our lead product ACP-01 for the treatment of CLI,” said Dr. Elmar Burchardt, Hemostemix president and CEO. “There is tremendous need for a therapy that reduces the number of limb amputations and improves survival. ACP-01 gives hope to patients who suffer from CLI and to physicians who lack other treatment options.”
About Critical Limb Ischemia (CLI)
CLI is a severe form of peripheral artery disease (PAD) caused by reduced blood flow to the legs. About half of CLI patients either die or require amputation of the affected limb within one year of diagnosis. Demand for a treatment is on the rise, as CLI predominately affects the growing population aged 50 and older.
For more information, email [email protected].
Neither the TSX Venture Exchange, Inc. nor its Regulation Service Provider (as that term is defined under the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
SanBio and Sunovion Recruiting for Phase 2b Trial for Cell Therapy Approach to Ischemic Stroke
On December 21, 2015, SanBio, Inc., and strategic partner Sunovion Pharmaceuticals announced they were recruiting patients for a Phase 2b clinical trial to evaluate the safety and efficacy of SB623 cells, a cell therapy product, for improving motor function in patients afflicted by ischemic stroke. When administered into neural tissue in laboratory settings (in vitro) and animal models, “SB623 cells have been shown to restore function to damaged neurons associated with stroke, traumatic brain injury, retinal diseases, and Parkinson’s disease.”
With 795,000 strokes happening in the U.S. each year and 15 million worldwide, there is major market potential for this cell therapy product.
For more information, see the full press release below, printed with permission from SanBio.
SanBio and Sunovion Begin Patient Recruitment for Phase 2b Trial to Test Regenerative Treatment for Chronic Stroke in North America
Studying patients impaired six months to five years following ischemic stroke
PRESS RELEASE – December 21, 2015; Mountain View, CA: SanBio, Inc., a scientific leader in regenerative medicine for neurological disorders, announced today that it has initiated patient recruitment for a Phase 2b clinical trial to further test the safety and efficacy of its proprietary cell therapy. SanBio is working closely with Sunovion Pharmaceuticals, Inc., a wholly owned subsidiary of Sumitomo Dainippon Pharma Co., Ltd., on the conduct of the clinical trial. Earlier clinical trial results suggested the therapy’s potential to improve motor function following an ischemic stroke. SanBio and Sumitomo Dainippon Pharma have entered into a joint development and license agreement for exclusive marketing rights in North America for SB623 for chronic stroke.
Ischemic strokes account for approximately 87 percent of all strokes in the United States and occur when there is an obstruction in a blood vessel supplying oxygen to the brain. With approximately 800,000 strokes occurring in the United States every year, stroke is the leading cause of acquired disability in the United States. Therapies for acute stroke generally do not result in meaningful improvement beyond the first six months following a stroke.
The ACTIsSIMA “Allogeneic Cell Therapy for Ischemic Stroke to Improve Motor Abilities” trial will examine the safety and efficacy of SB623 cells in patients who have experienced an ischemic stroke in the previous six months to five years and still suffer from motor impairments. SB623 cells are modified allogeneic mesenchymal stem cells, derived from bone marrow stromal cells isolated from healthy donors. When administered into neural tissue, SB623 cell therapy is designed to promote recovery from injury by triggering the brain’s natural regenerative ability.
Damien Bates, M.D., Chief Medical Officer & Head of Research at SanBio, said, “While existing drug treatments for stroke focus on prevention or acute care, there are no medical treatments currently available for people living with the debilitating effects of stroke, months or even years after the stroke occurred. We are optimistic that SB623 may provide a new treatment option for these patients.”
“This collaboration represents our shared commitment to patients with serious conditions where no effective drugs exist for whom regenerative therapies may offer a truly innovative therapeutic approach,” said Antony Loebel, M.D., Executive Vice President and Chief Medical Officer, Sunovion Pharmaceuticals Inc., and Head of Global Clinical Development for Sumitomo Dainippon Pharma Group.
The ACTIsSIMA Phase 2b clinical trial follows a Phase 1/2a clinical trial in which the SB623 cell treatment suggested improvements in motor function. The Phase 2b clinical trial will further evaluate the safety and efficacy of SB623 treatment. There are expected to be approximately 60 clinical trial sites throughout the United States, and total enrollment is expected to reach 156 patients.
About the ACTIsSIMA Trial
The ACTIsSIMA trial will examine the efficacy of SB623, modified adult bone-marrow-derived stem cells, when administered to patients with chronic motor deficit secondary to ischemic stroke. A secondary purpose is to evaluate the safety of SB623 in these patients.
About SanBio, Inc. (SanBio)
SanBio is a regenerative medicine company with cell based products in various stages of research, development and clinical trials. Its proprietary cell based product, SB623, is beginning Stage 2b clinical trials for treatment of chronic stroke. SanBio is expected to begin Stage 2 clinical trials for treatment of traumatic brain injury later in 2015. More information about SanBio, Inc. is available at www.san-bio.com.
About Sunovion Pharmaceuticals Inc. (Sunovion)
Sunovion is a leading pharmaceutical company dedicated to discovering, developing and commercializing therapeutic products that advance the science of medicine in the Psychiatry, Neurology and Respiratory disease areas to improve the lives of patients and their families. Sunovion, an indirect, wholly owned subsidiary of Sumitomo Dainippon Pharma Co., Ltd., is headquartered in Marlborough, Mass. More information about Sunovion Pharmaceuticals Inc. is available at www.sunovion.com.
About Sumitomo Dainippon Pharma Co., Ltd. (Sumitomo Dainippon Pharma)
Sumitomo Dainippon Pharma is a top-ten listed pharmaceutical company in Japan. Sumitomo Dainippon Pharma aims to produce innovative pharmaceutical products in the Psychiatry & Neurology area and the Oncology area, which have been designated as the company’s therapeutic focus areas. Sumitomo Dainippon Pharma was formed by the 2005 merger of Dainippon Pharmaceutical Co., Ltd., and Sumitomo Pharmaceuticals Co., Ltd. Today, Sumitomo Dainippon Pharma has about 7,000 employees worldwide. Additional information about Sumitomo Dainippon Pharma is available through its corporate website at www.ds-pharma.com.
Press Contact: Rebecca McRoberts
612-455-1912; [email protected]
Tisch MSRCNY to Launch Phase II Stem Cell Trial for Multiple Sclerosis (MS)

Dedicated to identifying the cause of multiple sclerosis (MS), the Tisch MS Research Center of New York was formally launched in 2006. However, it grew out of the MS center at the Neurological Institute of New York of the Columbia University Medical Center, a group which Dr. Saud Sadiq joined in 1992. [Read more…]
How 1.2 Trillion Online Searches Identified “Cell Therapy” as an Early-Stage Market
According to Internet Live Stat, Google now processes over 3.5 billion searches per day and 1.2 trillion searches per year. That is a massive amount of searches, especially when put into the context of there being approximately 7.4 billion people on earth.
Therefore, one of the single best resources for analyzing new market directions and the relative importance of specific geographic regions is Google Trends.1 In addition to reflecting online search behavior, Google Trends is an excellent tool for geographic analysis.
In the analysis below, the term “cell therapy” is searched within Google Trends to produce fascinating global findings.
Below, the number of online searches for the term “cell therapy” is shown, with the data plotted over a 10-year period. [Read more…]