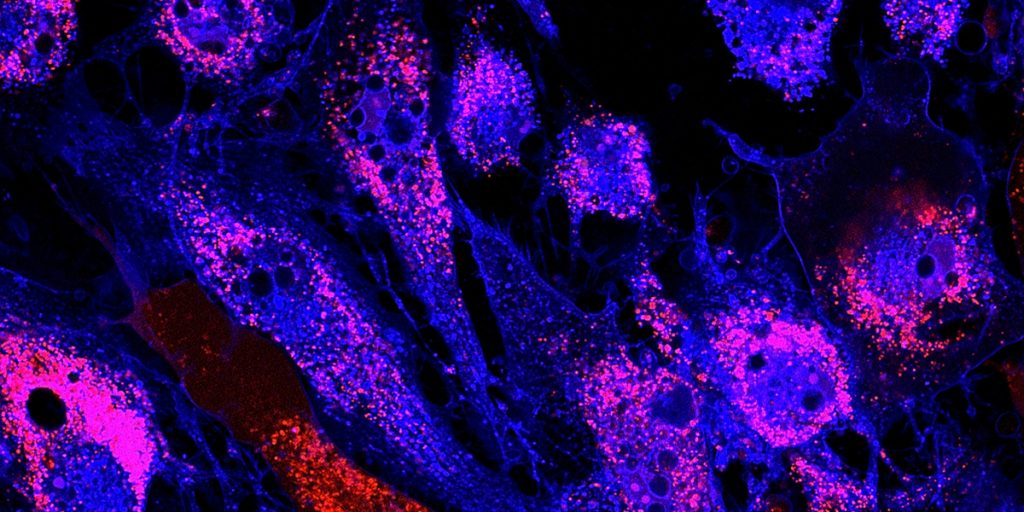
MSCs are a class of multipotent cells found in human tissues, such as bone marrow, adipose tissue, dental pulp, and periosteum, a layer of connective tissue that envelops the bones. They are also known as: Mesenchymal Stem Cells, Medicinal Signaling Cells, Mesenchymal Stromal Cells, and Marrow Stromal Cells. [Read more…]
Mesenchymal Stem Cells (MSCs)
Mesenchymal stem cells (MSCs) are a well-characterized population of adult stem cells that can differentiate into a variety of cell types (chondrocytes, osteoblasts, adipocytes, myocytes, and more).
The Current Status of Stem Cell Treatments for Osteoarthritis?
Are you exploring the current status of stem cell treatments for osteoarthritis, either for yourself or a loved one? Find out how stem cells are being used within osteoarthritis treatments in the article below.
In this article:
Who Is CryoHoldco? Latin America’s Largest Cord Blood Bank
CryoHoldco is a stem cell bank holding company that is the market leader in Latin America and one of the top 10 largest cord blood banks in the world. CryoHoldco is now seven times larger than any other stem cell bank in Latin America with over 275,000 stem cell units in storage.
Cord Blood Banking in Latin America – Interview with James Mendez of Cryoholdco
In this interview with James Alexis Mendez, we discuss the market strategy of CryoHoldco, a stem cell bank holding company that is the market leader in Latin America and one of the ten largest cord blood banks in the world. James Alexis Mendez currently serves as the CFO and Board member of CryoHoldco. Approaching 200,000 stem cell units in storage, CryoHoldco is more than 5X larger than any other stem cell bank in Latin America. CryoHoldco owns the cord blood market leaders in both Mexico and Colombia, and plans to expand into other regions of Latin America. [Read more…]
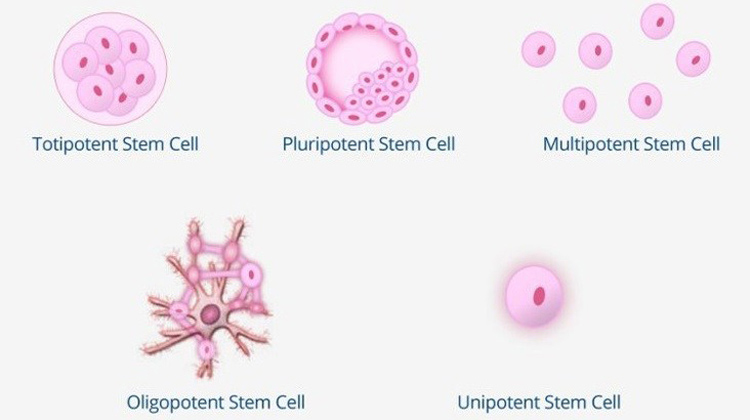
Do You Know The 5 Types Of Stem Cells?
As you start to learn about stem cells, one of the most common questions to have is, “What types of stem cells exist?” There is not an agreed-upon number of stem cell types, because one can classify stem cells either by differentiation potential (what they can turn into) or by origin (from where they are sourced).
This post is dedicated to explaining the five types of stem cells, based on differentiation potential. [Read more…]
- « Previous Page
- 1
- 2
- 3
- 4
- …
- 61
- Next Page »