If you’re an investor considering an opportunity within the induced pluripotent stem cell (iPSC) market, the technical aspects may initially appear to be confusing. However, if you focus on a few key points of understanding, you’ll be able to understand key attributes that define the cell type, as well as their advantages and disadvantages. [Read more…]
4 Major Areas of Commercialization for iPS Cells
Since the discovery of induced pluripotent stem cells in 2006, a great deal of basic research has been done to understand how to produce, manipulate, and utilize the stem cell type. In addition to this important basic research, a great deal of applied (“translational”) results has been done with the cell type. Induced pluripotent stem cells (also called iPS cells or iPSCs) are revolutionizing regenerative medicine because they represent a potential route for producing patient-specific stem cells for research or clinical use.
In the future, iPS cells will facilitate progress in personalized medicine by allowing a patient to use his or her own cells. In addition, iPSCs also show great promise in other areas, such as phamaco-toxicological screening, by allowing disease modeling and safety assessment of potential new drugs under development, in short, facilitating the study of a “disease in a dish.”
Levels of Repair Inducible with Induced Pluripotent Stem Cells (iPS Cells)
Induced pluripotent stem cells can be strategically utilized at several biological levels, as described below. [Read more…]
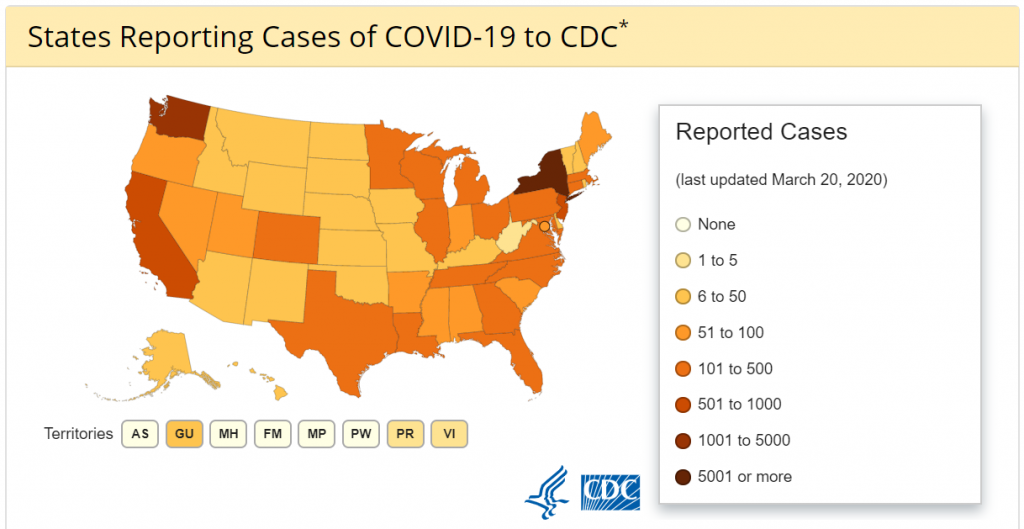
Could Cynata’s Cymerus™ Platform Be A Tool in the War Against Coronavirus?
What is the Cymerus™ technology and how could it potentially assist in the battle against Coronavirus cases on a global basis? Cymerus™ is a stem cell manufacturing platform being developed by Cynata Therapeutics Limited (ASX: CYP), an Australian regenerative medicine company.
The proprietary technology utilizes induced pluripotent stem cells (iPSCs) originating from an adult donor as the starting material for generating mesenchymoangioblasts (MCAs). It then differentiates these cells into mesenchymal stem cells (MSCs). [Read more…]
iPS Cell Therapy: Is Japan the Market Leader?
Although there are key players in multiple geographies worldwide, Japan has positioned itself as a hub for induced pluripotent stem cell (iPS cell) technology. iPS cells are made by reprogramming adult cells back into an embryonic-like state. Derived from skin or blood cells, they are not controversial.
- « Previous Page
- 1
- 2
- 3
- 4
- …
- 15
- Next Page »