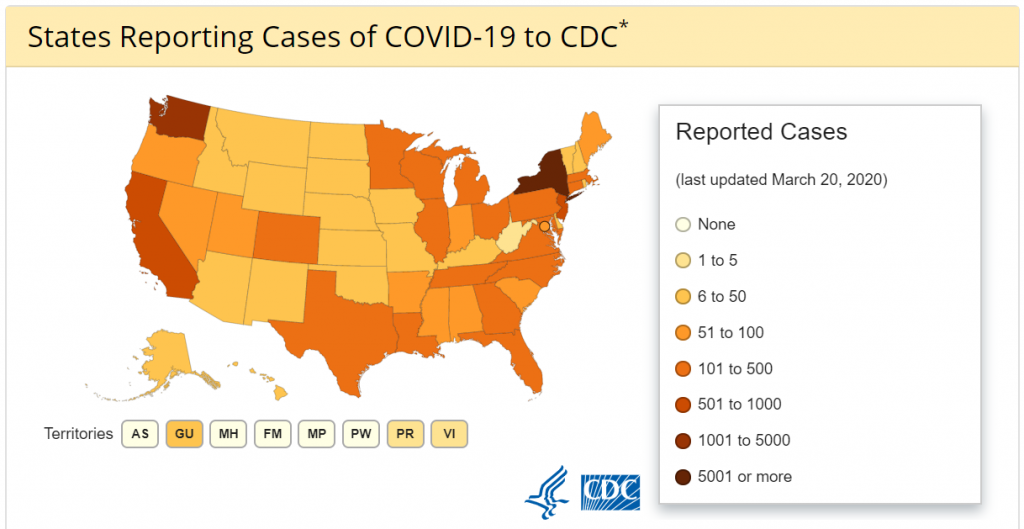
What is the Cymerus™ technology and how could it potentially assist in the battle against Coronavirus cases on a global basis? Cymerus™ is a stem cell manufacturing platform being developed by Cynata Therapeutics Limited (ASX: CYP), an Australian regenerative medicine company.
The proprietary technology utilizes induced pluripotent stem cells (iPSCs) originating from an adult donor as the starting material for generating mesenchymoangioblasts (MCAs). It then differentiates these cells into mesenchymal stem cells (MSCs). [Read more…]