Circulation Research, the official journal of the American Heart Association, has highlighted a novel stem cell therapy approach developed by BioCardia. For those not familiar with BioCardia, the company specializes in developing regenerative biologic therapies to treat cardiovascular disease.
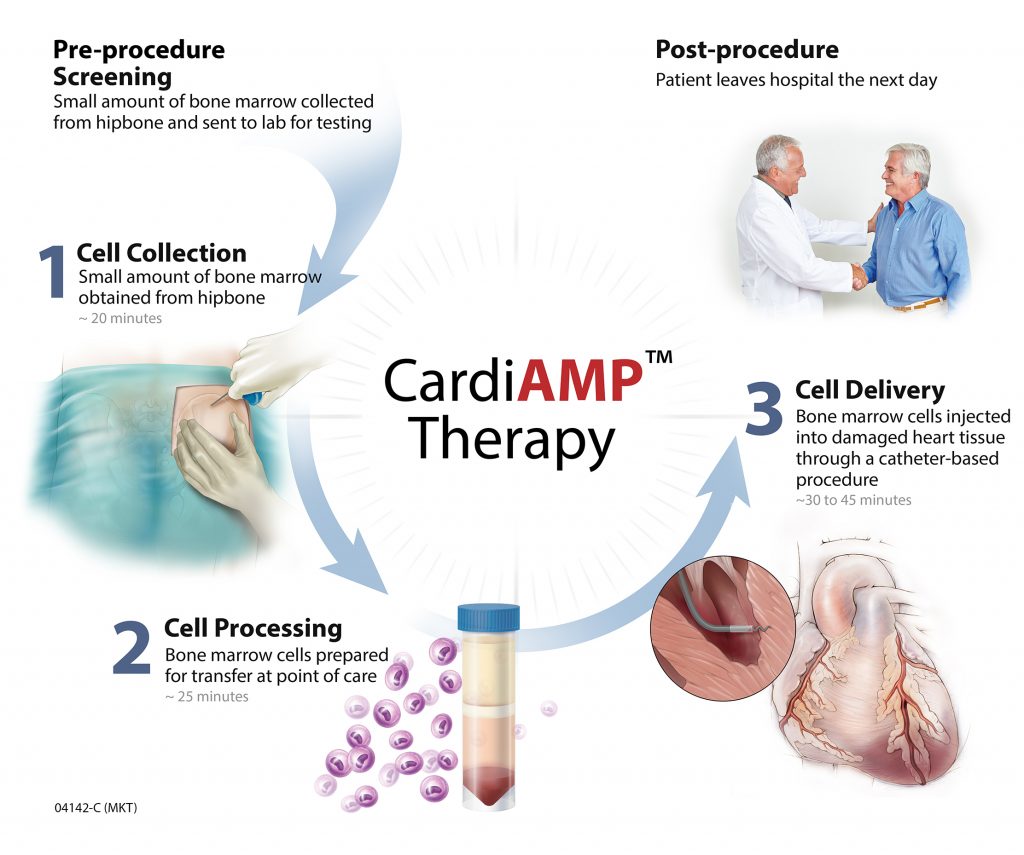
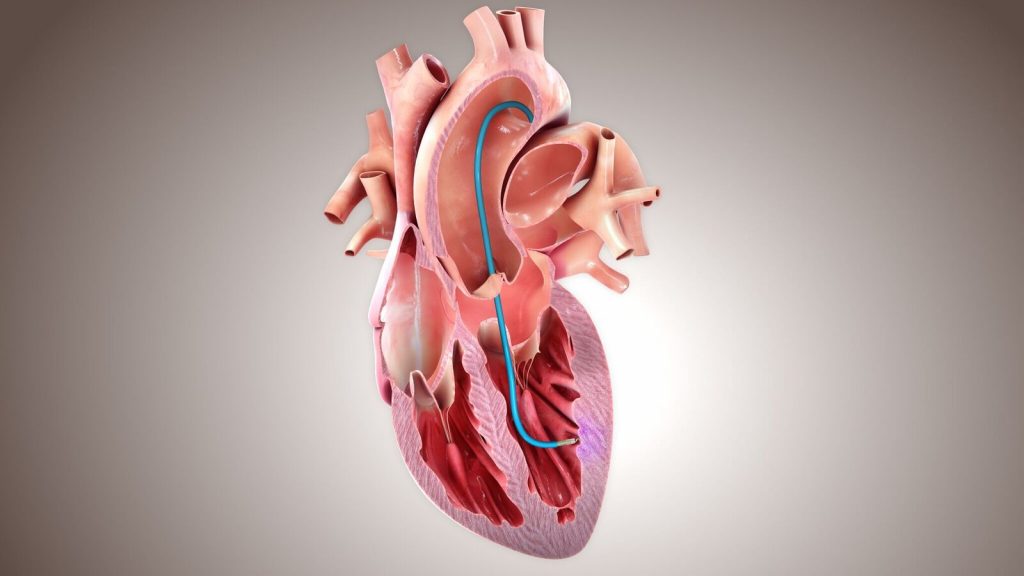
BioCardia has developed a stem cell technology that includes a novel method of administration, as well as a pre-procedure assay to achieve a comprehensive approach to heart failure. BioCardia’s CardiAMP and CardiALLO cell therapies are the Company’s biotherapeutic product candidates in clinical development.
In addition to providing Phase III 10-patient roll-incohort data for BioCardia’s CardiAMP device, the paper published by Circulation Research offers a comprehensive rationale of CardiAMP’s development as a treatment for heart failure. [Read more…]
 SAN CARLOS, Calif.
SAN CARLOS, Calif.















