 GERMANTOWN, Md., Nov. 12, 2018 — SERAXIS Inc., a rapidly growing regenerative medicine company today announced the publication of important trial considerations for SR-01, its pluripotent stem cell-derived islet therapy for insulin-dependent diabetes.
GERMANTOWN, Md., Nov. 12, 2018 — SERAXIS Inc., a rapidly growing regenerative medicine company today announced the publication of important trial considerations for SR-01, its pluripotent stem cell-derived islet therapy for insulin-dependent diabetes.
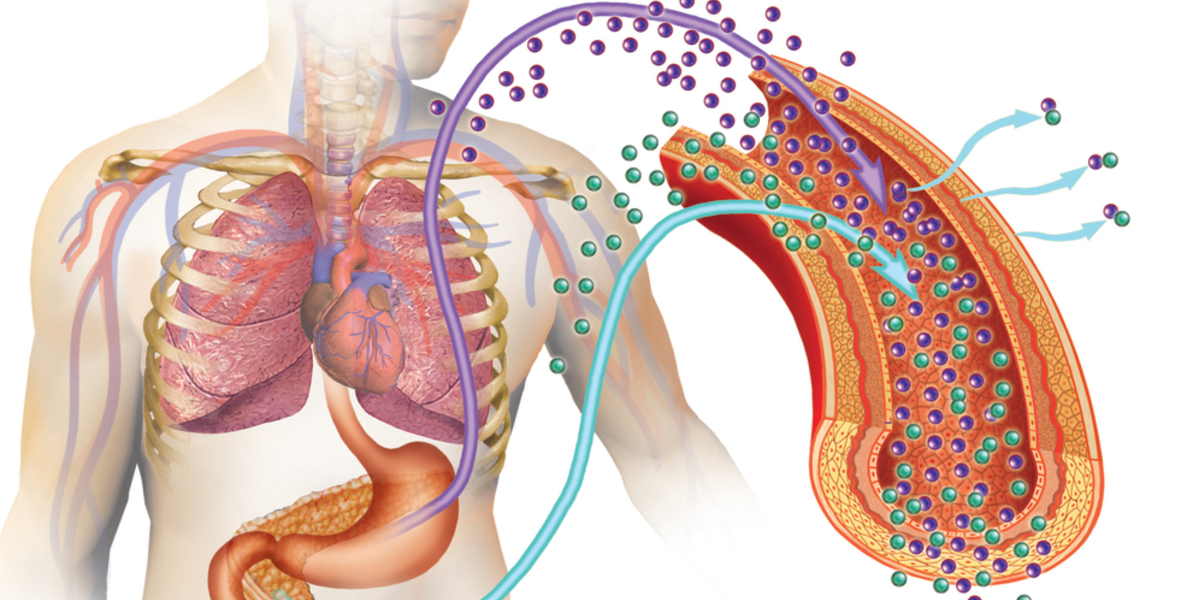
An analysis of islet transplant trials registered on clinicaltrials.gov was conducted with the goal of extracting insights to apply in the study design of SERAXIS lead candidate, SR-01 for the treatment of type 1 diabetes. The results of this analysis were published this week in Stem Cells Translational Medicine (doi:10.1002/sctm.18-0156). SR-01 is a combination therapy of insulin-producing cells encapsulated in the retrievable biocompatible SeraGraftTM device, designed to prevent immune system access to the cells after implantation.
The SeraGraft TM device has been shown to protect encapsulated cells in immune-competent diabetic models, to facilitate rapid vascularization of the graft without inducing a fibrotic response. If successful, SR-01 cell replacement therapy has the potential to provide a novel therapeutic modality to insulin-dependent type 1 diabetes patients, and SERAXIS is establishing a strategic relationship with a major clinical transplant program toward that end. The SERAXIS therapy could overcome some of the challenges associated with current pancreatic islet transplant protocols, providing a theoretically unlimited supply of insulin-producing cells and reducing or eliminating the need for post-transplant pharmacologic immunosuppression.
About SERAXIS INC.
SERAXIS is a privately held biotechnology company, incorporated in Singapore and the United States. SERAXIS used proprietary technologies to develop: (1) a cell therapy that functions shortly after transplantation in diabetic animals and; (2) a device, SeraGraft TM that safely protects these human pancreas cells from the immune system of animal hosts without need for pharmacologic immunosuppression. Further information can be found at www.seraxis.com
SOURCE SERAXIS Inc.
Do you have questions about Seraxis’ cell therapy replacement for diabetes? Ask them in the comments below.
Up Next: 15 Companies Developing Cell Therapy Treatments For Diabetes



















Tell Us What You Think!