|
|
Stem cells are primitive cells found in all multi-cellular organisms that are characterized by self-renewal and the capacity to differentiate into mature cell types. Stem cell research and experimentation have been in process for well over five decades, as stem cells have the unique ability to divide and replicate repeatedly.
In this article:
Stem Cell Applications
In a developing embryo, stem cells can differentiate into all of the specialized embryonic tissues. In adult organisms, stem and progenitor cells act as a repair system for the body, replenishing specialized cells and influencing the microenvironment through paracrine signaling.
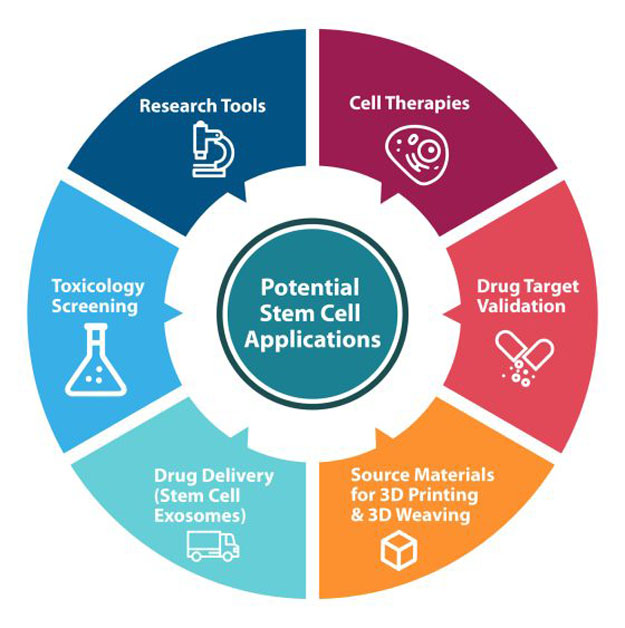
Over the past several decades, six powerful stem cell applications have emerged, including the use of stem cells for:
- Research Tools – Use of stem cells as research tools within a laboratory setting
- Cell Therapies – Use of stem cells as cellular therapeutics within the human body, mediated via cellular regeneration, paracrine signaling, or modulation of the microenvironment
- Source Materials for 3D Printing and 3D Weaving – Use of stem cells within 3D printing applications, including 3D printing of tissues/organs that are seeded with living cells or integration of stem cells into 3D printing inks
- Drug Target Validation – Validation of the predicted target using tissue-specific stem cell-derived cells
- Drug Delivery – Delivery of therapeutic products via stem cells and stem cell exosomes
- Toxicology Screening – Use of stem cells to evaluate effects of drugs on biological systems
List of Stem Cell Uses
Below is a more detailed explanation of each of these stem cell applications:
1. Research Tools
Stem cells serve as invaluable research tools in laboratories for understanding fundamental biological processes, disease mechanisms, and drug discovery. They offer a unique platform for studying cellular differentiation, development, and disease modeling. Researchers can manipulate stem cells to differentiate into various cell types, providing insights into tissue development and pathogenesis. Additionally, stem cells can be genetically modified to create disease models, enabling the study of disease progression and screening potential therapeutics.
2. Cell Therapies
Stem cells hold great promise in regenerative medicine for treating a wide range of diseases and injuries. They can differentiate into specialized cell types and replace damaged or dysfunctional cells within the body. Stem cell therapies involve the transplantation of either autologous (patient’s own) or allogeneic (donor-derived) stem cells to restore tissue function. These therapies can promote tissue repair, modulate immune responses, and stimulate endogenous regeneration through paracrine signaling and the secretion of growth factors. Examples of stem cell-based therapies include bone marrow transplants for hematopoietic disorders and mesenchymal stem cell injections for orthopedic injuries.
3. Source Materials for 3D Printing and 3D Weaving
Stem cells are integral to the field of bioprinting, where they are used to create bioinks for fabricating complex tissues and organs. By incorporating stem cells into bioink formulations, researchers can print anatomically accurate structures with cellular functionality. This approach enables the fabrication of tissue constructs for transplantation, disease modeling, and drug testing. Stem cells can also be integrated into scaffolds for 3D weaving applications, providing a framework for tissue regeneration and repair. This technology holds promise for creating patient-specific implants and tissue-engineered constructs.
4. Drug Target Validation
Tissue-specific stem cell-derived cells are valuable tools for validating drug targets and assessing drug efficacy. By generating differentiated cells from stem cells that mimic specific tissues or organs, researchers can screen potential therapeutic compounds in a relevant physiological context. This approach improves the predictive accuracy of preclinical drug testing and helps identify promising drug candidates for further development. Stem cell-based assays can elucidate drug mechanisms of action, identify off-target effects, and accelerate the drug discovery process.
5. Drug Delivery
Stem cells and their secreted extracellular vesicles, known as exosomes, offer unique advantages for drug delivery applications. Stem cells possess inherent homing abilities, allowing them to migrate to sites of injury or disease within the body. By loading therapeutic agents into stem cells or exosomes, researchers can target specific tissues or organs and enhance drug delivery efficiency. Stem cell-based delivery systems offer controlled release kinetics, prolonged circulation times, and reduced immunogenicity compared to conventional drug delivery methods. This approach shows promise for treating various diseases, including cancer, cardiovascular disorders, and neurological conditions.
6. Toxicology Screening
Finally, stem cells offer a versatile platform for evaluating the toxicological effects of drugs and environmental chemicals on biological systems. Traditional methods of toxicity testing often rely on animal models or immortalized cell lines, which may not accurately recapitulate human physiology or predict human responses. Stem cell-based assays provide a more physiologically relevant and human-specific approach to toxicology screening.
Stem cells can be differentiated into various cell types representing different tissues and organs, allowing researchers to create human-relevant models for toxicity testing. For example, induced pluripotent stem cells (iPSCs) can be directed to differentiate into hepatocytes (liver cells), cardiomyocytes (heart muscle cells), neurons (nerve cells), and other cell types affected by toxins.
Stem cell-based assays have the potential to improve regulatory compliance and reduce the reliance on animal testing in toxicology studies. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are increasingly encouraging the use of human-relevant models in toxicity testing to enhance the safety assessment of pharmaceuticals, chemicals, and consumer products.
Are there other potential applications that should be added to this list? Comment below and share your thoughts.




















Intranasal stem cells, immune cells (T cells, NK cells, etc.), microglia and macrophages bypass the blood-brain barrier to treat brain tumors, Parkinson’s, Alzheimer’s, stroke, MS and other brain disorders.
Together with my collaborators in Germany, especially Lusine Danielyan M.D., we discovered and patented (1) that therapeutic cells, including adult stem cells, immune cells (Treg, CAR-T, NK cells) and genetically-engineered cells, can be delivered to the brain using the noninvasive intranasal delivery method that I developed (2, 3). The successful treatment of Parkinson’s disease in an animal model using intranasal adult bone marrow derived mesenchymal stem cells has been reported (4). Intranasal stem cells bypass the blood-brain barrier to target the brain by traveling extracellularly along the olfactory neural pathway with minimal delivery to other organs. Once in the brain, adult stem cells target the damaged areas of the brain specifically to treat the underlying disease (4). Researchers at University Medical Center Utrecht in the Netherlands have demonstrated the effectiveness of intranasal stem cell treatment in an animal model of neonatal cerebral ischemia (5), neonatal stroke (6) and also in animals with neonatal hypoxia-ischemia brain damage (7-11) and with subarachnoid (12) and intracerebral hemorrhage (13). Researchers in Switzerland have demonstrated intranasal umbilical cord-derived stem cells preserve myelination in perinatal brain damage (14). Researchers at Emory University have used our intranasal stem cell treatment successfully in animal models of stroke (15) and neonatal stroke (16), and researchers at Uppsala University in Sweden have demonstrated that intranasal CAR/FoxP3-engineered T regulatory cells efficiently suppressed ongoing inflammation in an EAE model of multiple sclerosis leading to reduced disease symptoms (17). Intranasal adult neural stem cells have also been shown to improve the EAE model of multiple sclerosis (MS) (18) as have intranasal mesenchymal stromal cells (19). Other researchers have reported that intranasal stem cells target and treat brain tumors (20-23). This intranasal delivery, targeting and treatment technology can make stem cell treatments practical for brain disorders by eliminating the need for invasive neurosurgical implantation of cells and by eliminating the need for intravenous delivery that disperses cells throughout the body resulting in unwanted systemic exposure. This delivery and treatment method can facilitate the development of stem cell, immune cell, microglia, macrophage and genetically-engineered cell therapies for Parkinson`s, Alzheimer`s (24), MS, epilepsy, stroke, neonatal ischemia, brain tumors, traumatic brain injury and spinal cord injury (25).
In humans, Gonadotropin-releasing hormone expressing neurons are known to reach the brain by using this same olfactory neural pathway during development. In addition, pathologic cells, such as the amoeba Naegleria fowleri, are known to enter the brains of humans by this same pathway and cause amoebic infection of the brain. We have discovered how to use this pathway to deliver and target therapeutic cells and other biologics to the brain to treat brain disorders (26).
References:
1. Frey, Danielyan and Gleiter (2012). Methods, pharmaceutical compositions and articles of manufacture for administering therapeutic cells to the animal central nervous system. U.S. Patent 8283160 B2 filed 2009 and issued October 9, 2012.
2. Frey, W.H. 2nd (1997). Method of administering neurologic agents to the brain. US Patent 5,624,898 filed 1989 and issued April 29, 1997.
3. Danielyan, L., et al., Intranasal delivery of cells to the brain. Eur J Cell Biol, 2009. 88(6): p. 315-24.
4. Danielyan, L., et al., Therapeutic efficacy of intranasally delivered mesenchymal stem cells in a rat model of Parkinson disease. Rejuvenation Res, 2011. 14(1): p. 3-16.
5. van Velthoven, C. et al. Nasal administration of stem cells: a promising novel route to treat neonatal ischemic brain damage. Pediatr Res 2010. 68(5): p. 419-422.
6. van Velthoven, C. et al. Mesenchymal Stem Cell Transplantation Attenuates Brain Injury After Neonatal Stroke. Stroke 2013. 44:(5):1426-1432.
7. Donega, V., et al., The endogenous regenerative capacity of the damaged newborn brain: boosting neurogenesis with mesenchymal stem cell treatment. J Cereb Blood Flow Metab, 2013. 33(5): p. 625-34.
8. Donega, V., et al., Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol, 2014. 261: p. 53-64.
9. Donega, V. et al., Intranasal mesenchymal stem cell treatment for neonatal brain damage: long-term cognitive and sensorimotor improvement. PLoS ONE. 2013. 8(1):e51253.
10. Donega, V. et al., Intranasal Administration of Human MSC for Ischemic Brain Injury in the Mouse: In Vitro and In Vivo Neuroregenerative Functions. PLoS ONE 2014. 9(11): e112339.
11. Donega V. et al., Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr Res., 2015. 78(5):520-6.
12. Kooijman, E., et al., Intranasal Mesenchymal stem cell transplantation restores brain damage, improves sensori-motor function and reverses depressive-like behavior in a model of subarachnoidal hemorrhage in rats. Brain, Behavior, and Immunity. 2013, 32 (Supplement):e38.
13. Sun, J et al. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol., 2015 Oct;272:78-87.
14. Oppliger, B. et al. Intranasal Delivery of Umbilical Cord-Derived Mesenchymal Stem Cells Preserves Myelintation in Perinatal Bran Damage. Stem Cells and Development. 2016. 25(16):1234-1242.
15. Wei N., et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplantation, 2013. 22(6) p. 977-991.
16. Wei Z., et al. Intranasal Delivery of Bone Marrow Mesenchymal Stem Cells Improved Neurovascular Regeneration and Rescued Neuropsychiatric Deficits After Neonatal Stroke in Rats. Cell Transplantation, 2015. 24: 391-402.
17. Fransson M., et al. CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation, 2012. 9:112.
18. Wu, S., et al., Intranasal Delivery of Neural Stem Cells: A CNS-specific, Non-invasive Cell-based Therapy for Experimental Autoimmune Encephalomyelitis. J Clin Cell Immunol, 2013. 4:310.
19. Fransson, M., et al., Intranasal delivery of central nervous system-retargeted human mesenchymal stromal cells prolongs treatment efficacy of experimental autoimmune encephalomyelitis. Immunology, 2014. 142: p. 431–441.;
20. Reitz, M., et al. Intranasal delivery of neural stem/progenitor cells: A noninvasive passage to target intracerebral glioma. Stem Cells Trans Med, 2012. 1(12): p. 866-73.
21. Balyasnikova, I., et al., Intranasal Delivery of Mesenchymal Stem Cells Significantly Extends Survival of Irradiated Mice with Experimental Brain Tumors. Molecular Therapy, 2014. p. 22(1):140-8.
22. Mangraviti A, et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials, 2016, 100: 53-66.
23. Dey, M. et al., Intranasal Oncolytic Virotherapy with CXCR4-Enhanced Stem Cells Extends Survival in Mouse Model of Glioma. Stem Cell Reports, 2016. 7(3):471-82.
24. Danielyan, L. et al., Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014, 23 Suppl 1:S123-39.
25. Ninomiya, K. et al., Intranasal delivery of bone marrow stromal cells to spinal cord lesions. J Neurosurg Spine, 2015. 23(1):111-119.
26. Williams, G.S., Intranasal Drug Delivery Bypasses the Blood-Brain Barrier. Neurology Reviews, 2016. 24(4):1, 40-41.