Author: Jean-Philippe Richard, Ph. D. ([email protected])
Parkinson’s Disease (PD) affects some 10 million people worldwide, most commonly in patients over the age of 50. PD is associated with the loss of dopaminergic neurons in the substantia nigra region of the brain, leading to the characteristic tremors in the extremities, among other symptoms. There is a need for models of PD to help discover new therapies. To help address this need, we generated and characterized iPSC-derived PD dopaminergic neurons as a test case for functional neural differentiation.
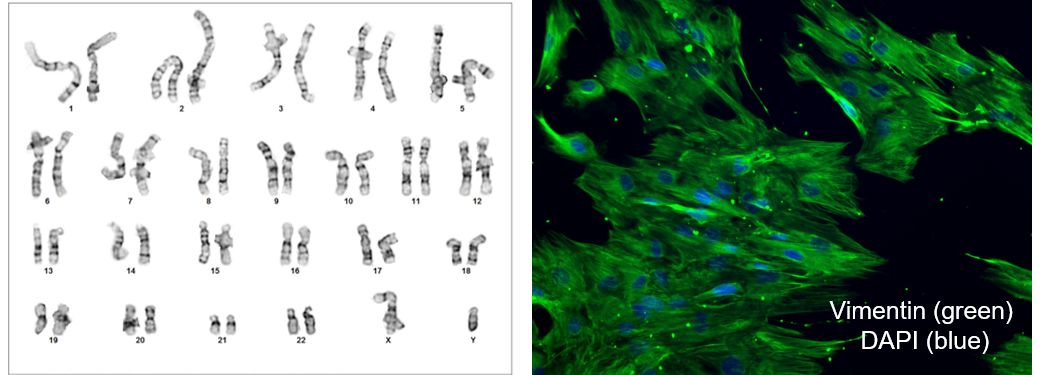
From our network of clinical sites, we ethically procured a skin punch biopsy from a medically diagnosed PD patient, along with clinical data and proper donor’s informed consent, all under the guidance of our IRB. The source material for this study is dermal fibroblasts derived from a skin punch biopsy. The fibroblast culture was subjected to a stringent quality control panel including karyotyping via G-banding, virology panel testing, sterility testing, Mycoplasma testing, species check, immunocytochemistry (ICC), and STR for identity tracking. (Figure 1). Viral pathogen, sterility and Mycoplasma were negative, and karyotyping analysis confirmed a male human chromosomal display. ICC confirmed the fibroblast nature of the culture.
After passing quality testing, the fibroblast culture was reprogrammed with our StemRNA 3rd Gen Reprogramming Technology, which uses a unique cocktail of non-modified RNAs, immune evasion mRNAs, and double-stranded miRNA. This mRNA reprogramming gives rise to reliably high-quality iPSCs with high efficiency (Figure 2). STR analysis confirmed that the iPSCs originated from the appropriate fibroblast donor.
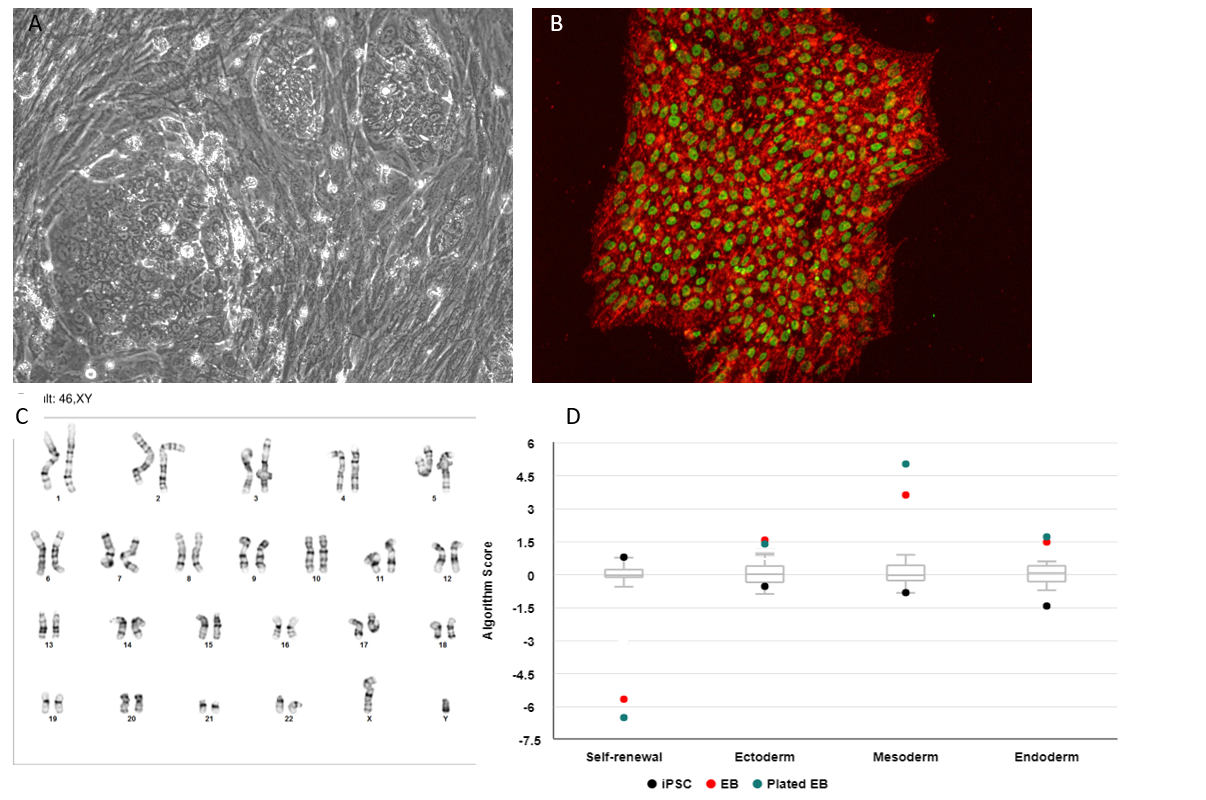
The quality control for the iPSC is extensive and includes mycoplasma testing, immunocytochemistry with four different specific stem cell markers (Nanog, TRA1-60, Oct4, and SSEA-4), karyotyping via g-banding, trilineage differentiation, TaqMan hPSC Scorecard Assay (ThermoFisher) and STR to confirm that the identity is the same as the primary tissue. Of notable interest, in all of our studies, our iPSC generated via mRNA reprogramming exhibit a higher level of stem cell gene expression in the TaqMan hPSC Scorecard Assay, compared to the internal control panel which is a blend of control embryonic stem cells and hiPSC.
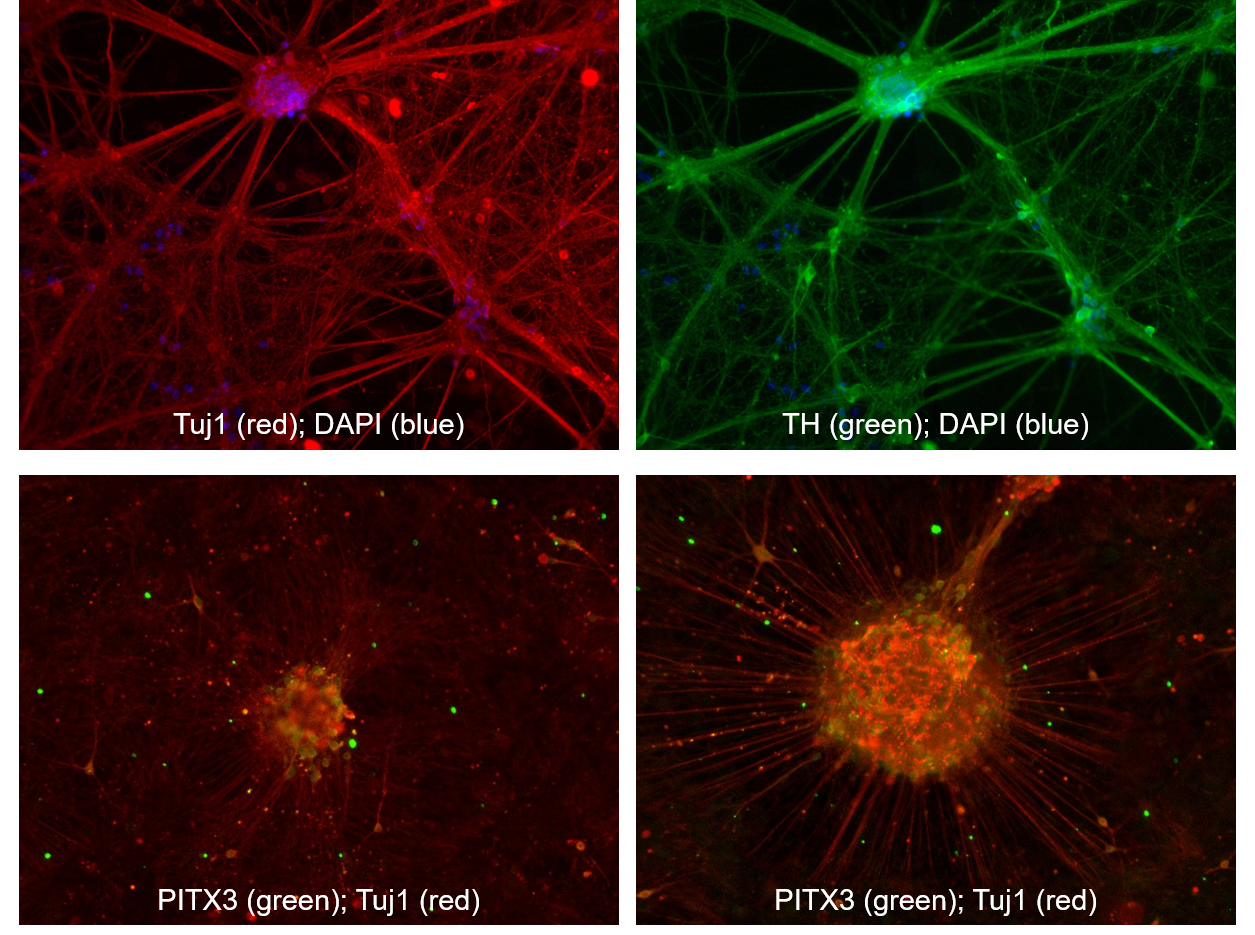
We differentiated the iPSC line from the Parkinson disease patient (PD iPSC line) alongside one of our commercial control iPSC line (CONTR iPSC line strain RPChiPS7713G1). We adapted the protocol from Kriks et al, Nature, 2011 and generated dopaminergic neurons from both lines. We confirmed the dopaminergic identity of our culture via immunocytochemistry. (Figure 3)
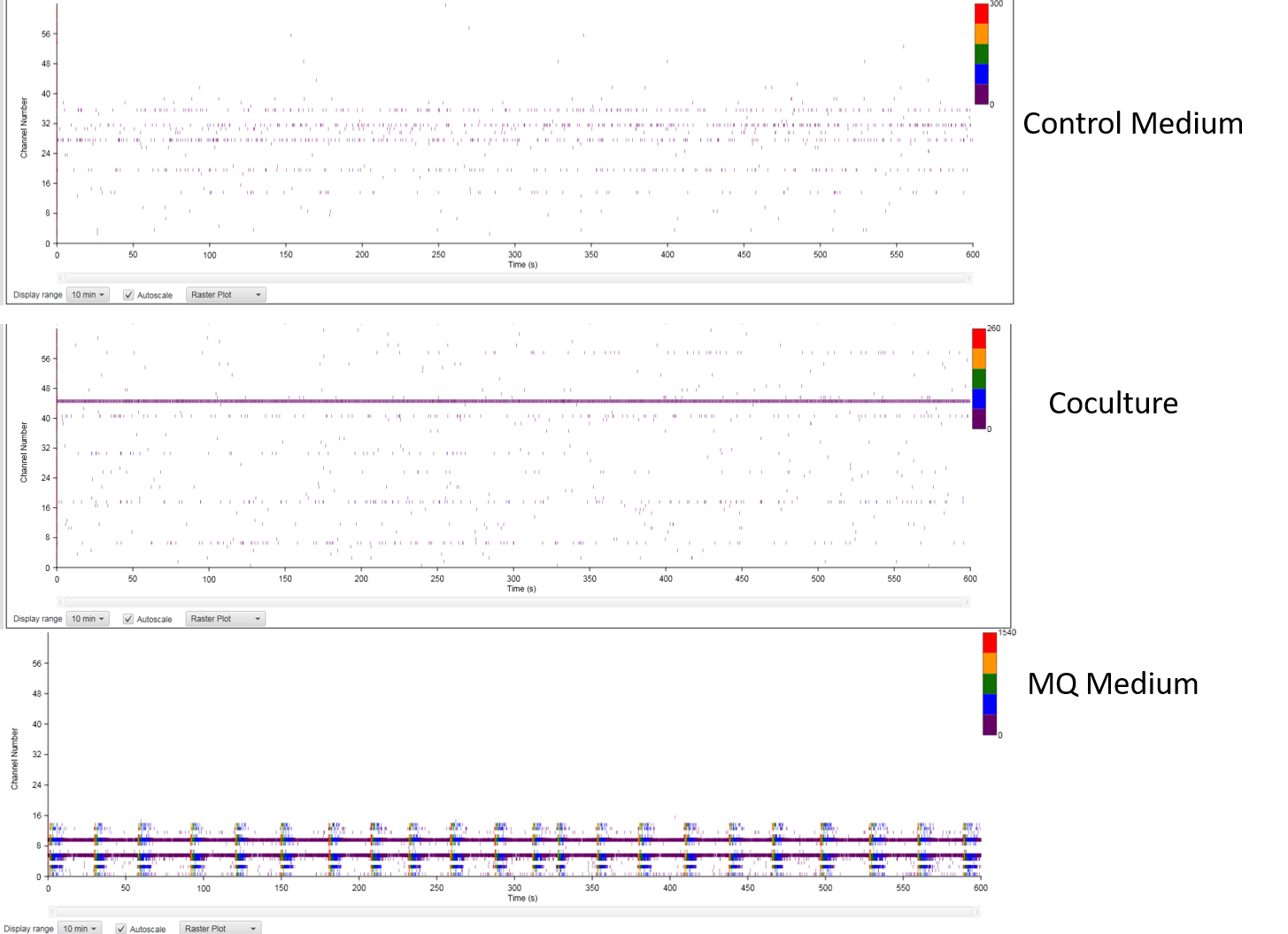
We matured our neuronal cultures using three different methods (Control Dopaminergic Medium, Neuro MQ Medium, and Astrocyte/ Neuron cocultures), showcasing how Neuro MQ Medium can help mature neurons efficiently and quickly. The advantage is we can preserve the purity of the neuronal culture, without having to introduce external cell lines and cell types to achieve the results expected for drug screening and disease modeling, as opposed to astrocyte/neuron co-culture systems.
Dopaminergic cultures were plated for MEA analysis using the MED64 MEA system from AlphaMed Scientific and cultured for 11 weeks under the three conditions before analysis (Figure 4). This study showed that the maturation of neuronal networks happens faster with Neuro MQ Medium than with control medium, and the maturation of dopaminergic neurons in Neuro MQ Medium is on par with the maturation of cocultures regarding the time of onset of individual spikes of electrical activity. Interestingly, in our study, the cocultures did not give rise to synchronous network bursts of electrical activity, where the cultures with Neuro MQ Medium exhibited strong synchronicity on week 10 and 11. Thus, Neuro MQ Medium is a solid option for a single subtype of neuronal network study.
We then decided to further assess the quality of the dopaminergic neurons after co-culture with astrocytes using RNA seq. Neurons derived from control and PD iPSCs were cultured in 24-well plates and matured for 60 days. A Transwell containing astrocytes was then added to the well above the neurons. The astrocytes were differentiated from the control iPSC line RPChiPS8023G1 and matured for 100 days. The Transwell set-up allows the exchange of fluids and media between two compartments with the advantage of no physical contact between the cells in the two compartments. Using this method, it was easy to collect the neuronal population for RNA seq without having the astrocyte population interfering in the analysis. The neurons were then isolated, and RNA was extracted and analyzed using RNA-seq.
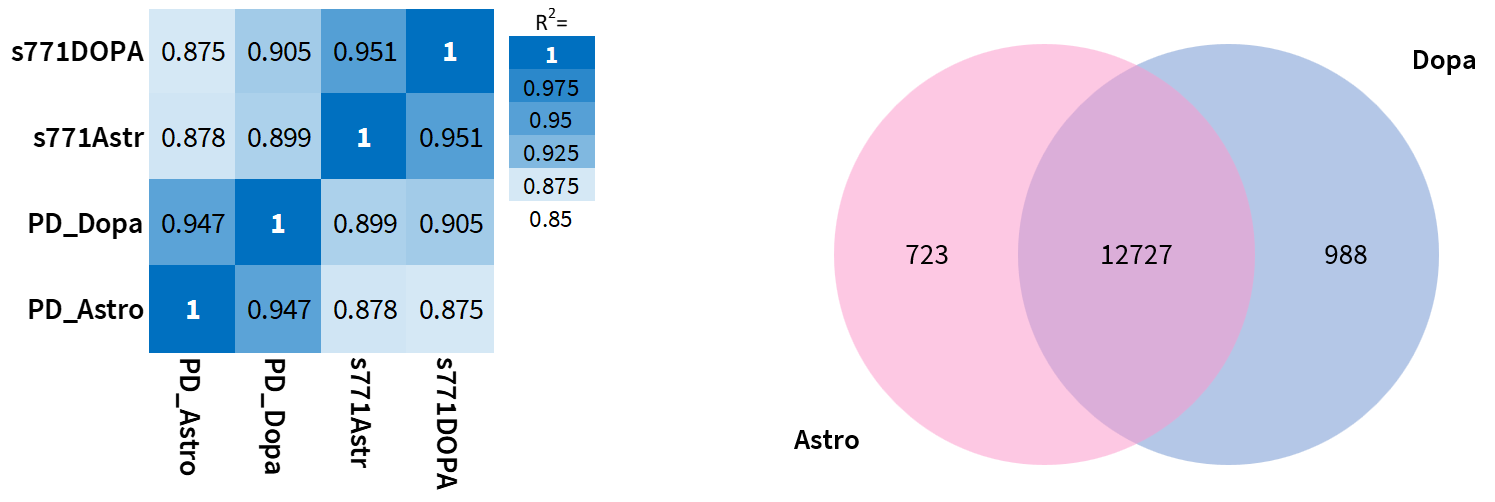
The analysis (Figure 5) revealed that the genetic background (control vs PD) is a bigger source of differences than is the method of maturation (control medium vs co-culture) as evidenced by the Pearson Correlation diagram which is a strong point to identify potential differences for disease modeling purposes. In addition, when comparing the differences between the two methods of differentiation using a Venn diagram, the number of genes in common is extensive (less than 10% are divergent). All RNA seq data was analyzed in collaboration with Navipoint Genomics.
In this study, we generated iPSCs from a Parkinson’s Disease patient’s fibroblasts and differentiated the iPSCs into dopaminergic neurons. Control dopaminergic neurons were generated from iPSCs derived from a healthy donor. We compared different culture conditions and identified NeuroMQ medium as an ideal medium for electrophysiological analysis. We examined the PD and control neurons by RNA-seq after coculture with astrocytes using a Transwell assay and showed that genetic differences (PD vs control) were a larger contributor to neuron diversity than was the culture method used. These results show that RNA-mediated reprogramming, followed by in vitro differentiation is ideal for generation of PD dopaminergic methods for further study.
Reprocell is a one-stop company for stem cell services, going from locating the donor of interest to primary cultures derivation, reprogramming into induced pluripotent stem cells (iPSC), and differentiation into the cellular subtype of interest. Of course, every step goes through a thorough and strict quality control process, all under the guidance of a dedicated Study Director.


















Exciting study from Reprocell. However, I’m curious about the percentage of Dopaminergic neurons achieved from starting iPS cell numbers. This is a really important aspect to not only make transplantations cost effective and realistic but also for disease modeling.
How do we intend to use these Ips derived dopoaminergic cells for the treatment of Parkinson’s Disease patient?
Thanks for your comment! From our company perspective, these cells are ready to be used for disease modeling and drug screening. For cell therapy, more in vivo work needs to be done, but these results are promising for animal model studies.
waiting for the reply
Thanks for your input and taking time to comment! Usually, we can achieve around 90 to 95% Tuj1 positive cells and of these, 40% to 60% are TH positive. It’s important to notice that the remaining 10% will most likely mature into astrocytes over time and we believe that the presence of control astrocytes would help the integration, maintenance and maturation of the dopaminergic neurons in vivo. It would definitely be interesting to investigate if maturation in vivo would change the ratio of dopaminergic neurons from progenitors.
Thanks for your input and taking time to comment! Usually, we can achieve around 90 to 95% Tuj1 positive cells and of these, 40% to 60% are TH positive. It’s important to notice that the remaining 10% will most likely mature into astrocytes over time and we believe that the presence of control astrocytes would help the integration, maintenance and maturation of the dopaminergic neurons in vivo. It would definitely be interesting to investigate if maturation in vivo would change the ratio of dopaminergic neurons from progenitors.