Within the United States, cord blood banks are subject to federal and state regulations and all cord blood banks must be registered with the U.S. FDA. The FDA governs all aspects of cord blood preservation, including the collection, processing, storage, labeling, packaging, and distribution of cord blood stem cells. In addition, there are a number of other agencies that accredit cord blood banks, including the AABB, Foundation for the Accreditation of Cellular Therapy (FACT), Clinical Laboratory Improvement Amendments (CLIA), and more.
Accredited Banks for Cord Bloods
In this article:
- Types of Accreditation for Cord Blood Banks
- AABB | Cord Blood Banking Accrediting Agency
- FACT and CLIA | Public and Private Cord Blood Banks
- International Cord Banking Accreditation
- AABB Accreditation: “Gold Standard” for Cord Blood Banking
- Gold Standard for Cord Blood Transplant, Harvesting, and Banking
- Criteria for Gold Standard Storage of Cord Blood
- Cord Blood Banking Cost for Accreditation
- Geographical Breakdown of AABB Accredited Cord Blood Banks
- 76 Accredited Banks for Cord Bloods
- AABB Accredited Cord Blood Banks Worldwide
Banks for Cord Bloods Worldwide
Types of Accreditation for Cord Blood Banks
There are two different standards which can apply: cGTP (current Good Tissue Practices) and cGMP (current Good Manufacturing Practices)
- cGTP standards apply to the collection, processing, and storage of human cells, tissues, and cellular/tissue-based products (HCT/Ps), and are regulated by the Center for Biologics Evaluation and Research.[1] All U.S. cord blood banks must be compliant with cGTP standards.
- cGMP standards apply to the manufacturing of a product that is considered a drug, and the determination for whether or not a cord blood bank must be compliant with cGMP standards is based upon the nature of the product that a facility manufactures. Because cGTP standards are based upon cGMP standards, there are many similarities between the two systems.
AABB | Cord Blood Banking Accrediting Agency
 In addition, there are a number of other agencies in the U.S. that accredit cord blood banks. The primary optional accrediting agency for cord blood banks is the AABB (formerly the American Association of Blood Banks). While optional, AABB accreditation is considered one of the leading global credentials that a cord blood bank can hold.
In addition, there are a number of other agencies in the U.S. that accredit cord blood banks. The primary optional accrediting agency for cord blood banks is the AABB (formerly the American Association of Blood Banks). While optional, AABB accreditation is considered one of the leading global credentials that a cord blood bank can hold.
FACT and CLIA | Public and Private Cord Blood Banks
The Foundation for the Accreditation of Cellular Therapy (FACT) and Clinical Laboratory Improvement Amendments (CLIA) are the two other most common accreditation agencies for U.S. cord blood banks, both public and private. ISO Certification is less common but is a type of management systems certification that ensures that statutory and regulatory requirements related to the product are met.
International Cord Banking Accreditation
For international companies, there are similar accreditations that are desirable to pursue. These include the EHA (European Hematology Association) accreditation, ISCGT (International Society for Cell & Gene Therapy of Cancer) accreditation, and TGA (Therapeutic Goods Administration) licensure, among others.
AABB Accreditation: “Gold Standard” for Cord Blood Banking
The AABB is a professional body and standards organization that was founded in 1947 as the American Association of Blood Banks. While based in the U.S., the organization is international in reach, with members in 80 countries. It has taken on a broader scope to include all of the transfusion, as well as cellular therapies, including ones based on hematopoietic stem cells. For this reason, AABB accreditation has great significance within the cord blood banking sector.[2]
Gold Standard for Cord Blood Transplant, Harvesting, and Banking
In 2005, the organization changed its name from the American Association of Blood Banks to AABB to reflect the changes in scope and operations. AABB accreditation assures donors and clients that a cord blood bank is delivering a quality process—from collection to storage, and, if necessary, transplantation. Cord blood banks electing to pursue accreditation by the AABB require a bi-annual audit to evaluate quality assurance guidelines and practices. AABB also publishes voluntary standards for cellular therapy product services, including cord blood banking. These standards augment, rather than replace, any federal or state requirements.
Criteria for Gold Standard Storage of Cord Blood
Their standards describe the minimum acceptable requirements for facilities providing these services and covering all aspects of the operation, including:
- A process for approval of vendors providing supplies
- The consent, donor screening, and collection processes
- Product qualification, testing, processing, storage, and release
- Equipment and facility maintenance
- A process for personnel selection and training
- A process to monitor and improve the quality of services
Cord Blood Banking Cost for Accreditation
At such time that a cord blood banking facility believes that it complies with these standards, it applies for accreditation by the AABB. This involves a detailed and lengthy application process during which the facility is assessed by a team experienced in the cord blood field. Any evidence of non-compliance with AABB standards is brought to the attention of the bank, and corrective action is taken before accreditation is granted. Accreditation is then awarded for a two-year period. As such, AABB accreditation is a stringent and costly accreditation standard.
Geographical Breakdown of AABB Accredited Cord Blood Banks
It is interesting to consider how many, as well as which specific cord blood laboratories, the AABB has accredited. It is also interesting to consider a geographical breakdown of these cord blood banks.
76 Accredited Banks for Cord Bloods
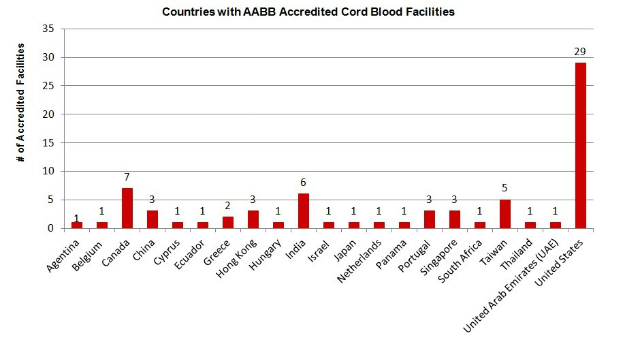
There are currently 76 AABB accredited cord blood banks worldwide, an increase of 9 facilities since January 1, 2014.[3] Of the 76 total accredited cord blood banks worldwide, 29 are located within the United States, again asserting the relatively mature nature of cord blood banking industry within the United States, as compared to other parts of the world.
The table below shows the 21 countries that currently have AABB accredited cord blood banks operating within them.
As of January 1, 2014, there were only 18 countries with accredited facilities, meaning three new countries joined the ranks during 2014.
TABLE. Countries Containing AABB Accredited Cord Blood Banks
| COUNTRY | # OF AABB ACCREDITED BANKS |
| Argentina | 1 |
| Belgium | 1 |
| Canada | 7 |
| China | 3 |
| Cyprus | 1 |
| Ecuador | 1 |
| Greece | 2 |
| Hong Kong | 3 |
| Hungary | 1 |
| India | 6 |
| Israel | 1 |
| Japan | 1 |
| Netherlands | 1 |
| Panama | 1 |
| Portugal | 3 |
| Singapore | 3 |
| South Africa | 1 |
| Taiwan | 5 |
| Thailand | 1 |
| United Arab Emirates (UAE) | 1 |
| United States | 29 |
Broken down by country, 38% of all AABB accredited cord blood facilities are located in the United States (29 facilities) and 9% are located within Canada (7 facilities). India also contains a substantial number (6 facilities), as does Taiwan (5 facilities).
The two geographic regions that have the highest rates of AABB accreditation are North America and Asia.
AABB Accredited Cord Blood Banks Worldwide
Finally, a full list of AABB accredited cord blood facilities worldwide is shown below.[4]
| ORGANIZATION | CITY | COUNTRY |
| Hospital Nacional de Pediatria Garrahan | Buenos Aires | Argentina |
| Cryo-Save Labs NV. | Niel | Belgium |
| Centro Regional de Hemoterapia do HCFMRP – USP – Hemocentro de Ribeirão Preto | Ribeirao Preto, Sao Paulo | Brazil |
| Cryopraxis Criobiologia LTDA | Rio De Janeiro | Brazil |
| CordVida | Sao Paulo | Brazil |
| Hospital Israelita Albert Einstein | Sao Paulo | Brazil |
| *HealthCord Cryogenics Corporation | Vancouver | Canada |
| Insception Lifebank Cord Blood Program, Insception Biosciences | Burnaby | Canada |
| Cells for Life Ltd | Markham | Canada |
| Create Cord Blood Bank | Toronto | Canada |
| Insception Lifebank Cord Blood Program, Insception Biosciences | Mississauga | Canada |
| Progenics Cord Blood Cryobank | Toronto | Canada |
| OVO Biosurance | Montreal | Canada |
| *Beijing Cord Blood Bank | Beijing | China |
| Boyalife Inc. | Wuxi | China |
| *Jiangsu Beike Bio-Technology Co., Ltd. | Jiangsu Province | China |
| *CBB Lifeline Biotech LTD | Nicosia | Cyprus |
| Biocells Discoveries Internacional S.A. | Quito, Pichincha | Ecuador |
| *Biohellenika S.A. | Thessaloniki | Greece |
| Medstem Services | Marousi 151 23, Athens | Greece |
| Cordlife (Hong Kong) Ltd. | Shatin, New Territories | Hong Kong |
| Cryolife Company Limited (CRYOLIFE) | Shatin | Hong Kong |
| HealthBaby Biotech (HK) Co, Ltd. | Shatin, N.T | Hong Kong |
| *KRIO Sejt-es Szovetbank Zrt. | Budapest | Hungary |
| Cryobanks International India Pvt. Ltd. | Gurgaon | India |
| Reliance Life Sciences | Navi Mumbai, Maharashtra | India |
| Lifecell International Pvt. Ltd., Chennai | Keelkottoiyur | India |
| StemCyte India Therapeutics Pvt. Ltd. | Gandhinagar | India |
| Lifecell International Pvt. Ltd., Manesar | Gurgaon | India |
| Cordlife Sciences India Pvt. Ltd. | 24 Paraganas (south) | India |
| Chaim Sheba Medical Center | Tel-Hashomer | Israel |
| *EIL, Inc. | Tokyo | Japan |
| Tissue Bank Cryo-Save (Stichting Cryo-Save) | Zutphen | Netherlands |
| Cordon de Vida | Panama | Panama |
| *The Polish Stem Cells Bank S.A. | Warsaw | Poland |
| *Crioestaminal | Cantanhede | Portugal |
| Cordlife Group Limited | Singapore | Singapore |
| *National University Hospital Pte Ltd. | Singapore | Singapore |
| Singapore Cord Blood Bank Limited | Singapore | Singapore |
| *Netcells Biosciences | Parklands, Gauteng | South Africa |
| Bionet Corporation | Taipei City | Taiwan |
| Bionet Corporation | Tainan City | Taiwan |
| HealthBanks Biotech Co, Ltd. | Taipei | Taiwan |
| StemCyte Taiwan Co. Ltd. | Lin-Kou, Taipei County | Taiwan |
| Taiwan Advance Bio-Pharm, Inc. | Shijr City, Taipei County | Taiwan |
| *Cryoviva (Thailand) Ltd. | Nakhon Pathom | Thailand |
| Cryo-Save Arabia | Dubai | United Arab Emirates |
| Celebration Stem Cell Centre | Gilbert | United States |
| Cord Blood Registry | Tucson | United States |
| California Cryobank Stem Cell Services, Inc, (DBA: Familycor) | Los Angeles | United States |
| CBR Systems, Inc. | San Bruno | United States |
| PacifiCord | Irvine | United States |
| San Diego Blood Bank | San Diego | United States |
| StemCyte Inc. | Covina | United States |
| University of Colorado Cord Blood Bank | Aurora | United States |
| Lifeline Cryogenics, LLC | Stamford | United States |
| Cord Use Cord Blood Bank | Orlando | United States |
| Cryo-Cell International Inc. | Oldsmar | United States |
| Lifeforce Cryobanks | Altamonte Springs | United States |
| Oneblood, Inc. – West Region | Saint Petersburg | United States |
| Stem Cell Cryobank | Boynton Beach | United States |
| ITxM – Lifesource Blood Services | Rosemont | United States |
| Cook General Biotechnology, LLC | Indianapolis | United States |
| Norton Healthcare, Inc. | Louisville | United States |
| ViaCord Processing Laboratory (VPL) | Hebron | United States |
| New England Cryogenic Center, Inc. | Newton | United States |
| Michigan Blood | Grand Rapids | United States |
| St. Louis Cord Blood Bank and Cellular Therapy Laboratory | Saint Louis | United States |
| Elie Katz Umbilical Cord Program | Montvale | United States |
| LifebankUSA/Celgene Cellular Therapeutics | Cedar Knolls | United States |
| New Jersey Cord Blood Bank | Montvale | United States |
| Progenitor Cell Therapy, LLC | Allendale | United States |
| Cord Blood America, Inc. | Las Vegas | United States |
| Cleveland Cord Blood Center | Cleveland | United States |
| South Texas Blood & Tissue Center | San Antonio | United States |
| Puget Sound Blood Center | Seattle | United States |
In your opinion, how has banking neonatal cord blood increasingly developed throughout the world? Share your thoughts in the comments section below.
About Us
BioInformant is the only research firm that has served the stem cell sector since it emerged. Our management team comes from a BioInformatics background – the science of collecting and analyzing complex genetic codes – and applies these techniques to the field of market research. BioInformant has been featured on news outlets including the Wall Street Journal, Nature Biotechnology, CBS News, Medical Ethics, and the Center for BioNetworking.
Footnotes
[1] FDA.gov, (2014). Tissue & Tissue Products. [online] Available at: http://www.fda.gov/BiologicsBloodVaccines/TissueTissueProducts/default.htm [Accessed 6 Nov. 2014].
[2] AABB.org, (2014). [online] Available at: http://www.aabb.org/about/who /Pages/default.aspx [Accessed 5 Nov. 2014].
[3] Aabb.org, (2014). AABB Accredited Cord Blood (CB) Facilities. [online] Available at: http://www.aabb.org/sa/facilities/celltherapy/Pages/CordBloodAccrFac.aspx [Accessed 2 Nov. 2014].
[4] Aabb.org, (2014). AABB Accredited Cord Blood (CB) Facilities. [online] Available at: http://www.aabb.org/sa/facilities/celltherapy/Pages/CordBloodAccrFac.aspx [Accessed 2 Nov. 2014].





















Tell Us What You Think!