
July 30, 2018 – Fresenius Medical Care, the world’s largest provider of dialysis products and services, announced today that its subsidiary Unicyte AG has achieved a key preclinical milestone in its regenerative medicine program for chronic kidney disease. The company was able to confirm a disease modifying potential for its proprietary nano-Extracellular Vesicles (“nEVs” are stem cell-derived particles that support communication between cells) in a second preclinical model of chronic kidney disease.
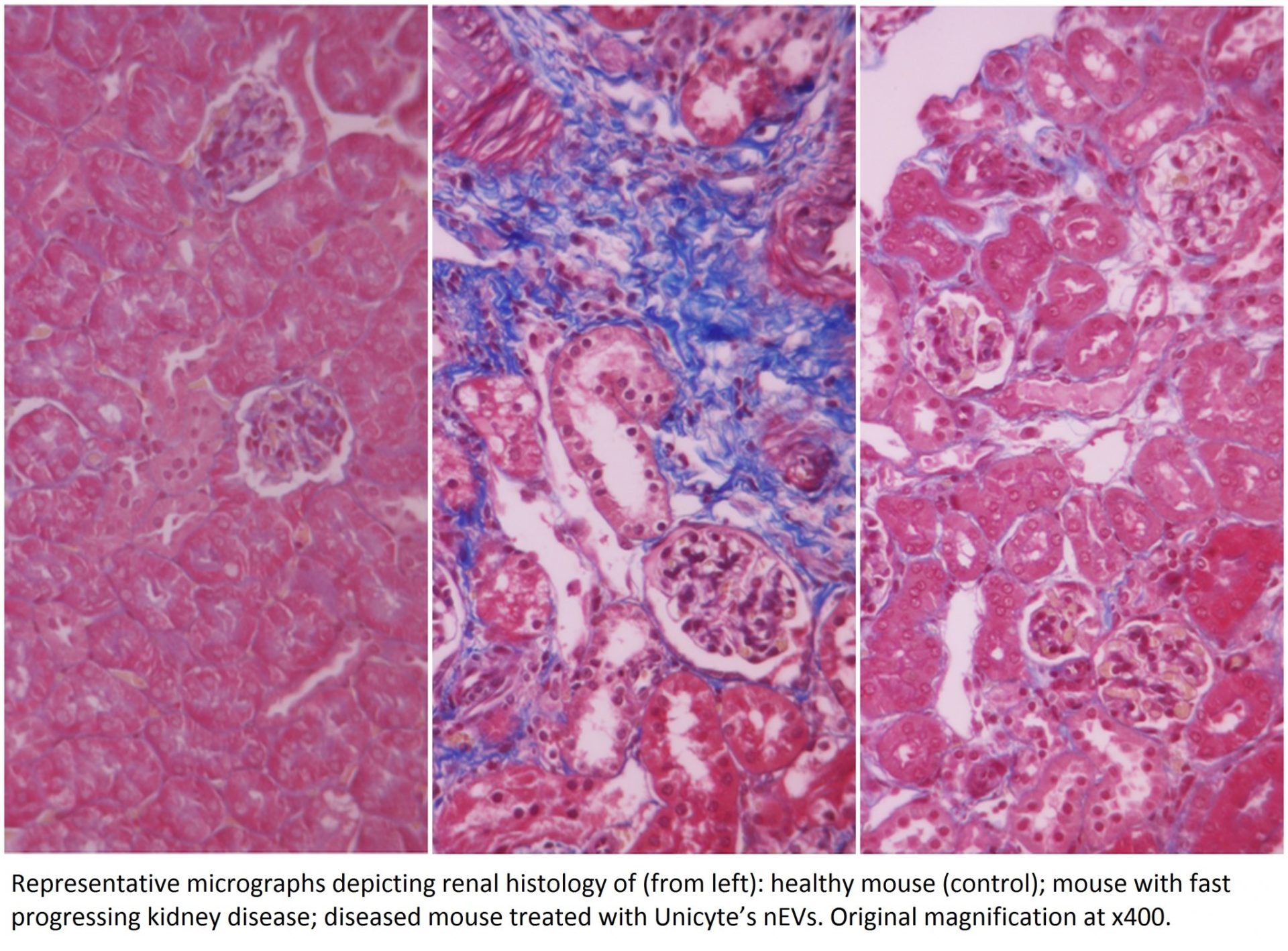
When administered to mice with fast progressing kidney disease, Unicyte’s nEVs prevented renal fibrosis, a hallmark of chronic kidney disease. In particular, nEVs significantly reduced interstitial fibrosis and tubular necrosis while also inhibiting infiltration of various cells. This resulted in near-to-normal recovery of kidney function. The study conducted in collaboration with Prof. Giovanni Camussi of the University of Turin, Italy, has been accepted for publication in the peer-reviewed journal Frontiers of Immunology (https://doi.org/10.3389/fimmu.2018.01639).
These new results support previous findings in a preclinical model of slowly progressing kidney disease (diabetic nephropathy), a major pathology that often leads to end-stage renal disease. Combined results of these studies demonstrate the efficacy and the underlying mechanism of action of nEVs in preventing renal fibrosis and subsequent progression to end-stage renal disease. Unicyte will continue the preclinical and clinical development of its proprietary nEVs for treatment of chronic and acute kidney diseases.
Dr. Olaf Schermeier, Fresenius Medical Care’s CEO for Global Research and Development, said: “We are very excited about the progress we have made with our research and development activities over the last 30 months since we have established Unicyte. Based on these achievements, Unicyte will continue to explore the potential of nEVs for the treatment of patients in pre-dialysis stages of chronic kidney disease.”
Prof. Giovanni Camussi, Professor Emeritus at the University of Turin and Member of Unicyte’s Scientific Advisory Board, said, “nEVs are a promising regenerative medicine technology platform. Our aim is to develop new and better treatment options for severely and chronically ill patients over time. Achieving this preclinical milestone represents an important step towards testing nEVs in the clinical setting.”
With multiple therapeutic programs at the clinical and preclinical stage, Unicyte has established a broad pipeline in kidney and liver diseases, diabetes and oncology. The company is seeking strategic partnerships for its non-renal programs.
Fresenius Medical Care is the world’s largest provider of products and services for individuals with renal diseases of which around 3.2 million patients worldwide regularly undergo dialysis treatment. Through its network of 3,790 dialysis clinics, Fresenius Medical Care provides dialysis treatments for 322,253 patients around the globe. Fresenius Medical Care is also the leading provider of dialysis products such as dialysis machines or dialyzers. Along with its core business, the company provides related medical services in the field of Care Coordination. Fresenius Medical Care is listed on the Frankfurt Stock Exchange (FME) and on the New York Stock Exchange (FMS).
For more information visit the company’s website at www.freseniusmedicalcare.com.
Unicyte AG is a regenerative medicine company with a focus on kidney & liver disorders, diabetes and oncology. Unicyte evolved from a long-term research collaboration of Italy’s University of Turin and Fresenius Medical Care. Unicyte is headquarted in Oberdorf NW, Switzerland, and is an independent affiliate of Fresenius Medical Care, the world’s largest provider of products and services for individuals with renal diseases.
For more information visit the company’s website at www.unicyte.ch.
Disclaimer
This release contains forward-looking statements that are subject to various risks and uncertainties. Actual results could differ materially from those described in these forward-looking statements due to certain factors, including changes in business, economic and competitive conditions, regulatory reforms, foreign exchange rate fluctuations, uncertainties in litigation or investigative proceedings, and the availability of financing. These and other risks and uncertainties are detailed in Fresenius Medical Care AG & Co. KGaA’s reports filed with the U.S. Securities and Exchange Commission. Fresenius Medical Care AG & Co. KGaA does not undertake any responsibility to update the forward-looking statements in this release.



















I want to thank you for all of you and your world Team researchers. I am a 61-year-old male I have never Had any type of a severe illness in my entire life. I take no medication’s medication‘s and never have for anything, No high blood pressure no high cholesterol levels no anything. I Have never been any sort of a health fanatic but have always ate fairly clean in my life. I was diagnosed with a type of kidney cancer and surprise as most people I’m sure are. Your publications on the forward treatments of this disease give me hope that there are other forms of treatment for this disease considering conventional chemo and radiation cannot be performed on my particular type of cancer where it is located, for fear from the medical field that it may only heighten the problem. Even though my physicians were probably not supposed to guide me in a different area outside of Western medicine because of the conglomerates that they work for I am fortunate to have physicians that have been my lifelong friends. They steered me in the direction of stem cells and I have decided to go with this treatment since The alternative treatment based on Western Has not worked due to my body rejecting rejecting the drugs. Thank you for all of your encouraging articles you give all of them people around the world that read these articles rejuvenated hope.
Thank you again
Sincerely Herman Hook