We have helped hundreds of the top companies increase revenue from stem cell products and decrease risk. The key is targeted market intelligence, because it will help you to approach institutional investors for fundraising rounds, guide internal decision-making within your company, and reduce the risk that has traditionally accompanied new product development. Simply put, it will help you make smarter decisions, faster.
Whether or not you can invest in the recently released global strategic report, “Stem Cell Research Products – Opportunities, Tools, and Technologies,” the following is an important introduction to the stem cell research products marketplace, including key trends and future projections.
The Market for Stem Cell Research Products
Stem cells are still a relatively new discovery, as the first mouse embryonic stem cells were derived from embryos in 1981 by Martin Evans, Matthew Kauffman, and Gail Martin,[1],[2] but it was not until 1995 that the first successful culturing of embryonic stem cells from non-human primates occurred at the University of Wisconsin-Madison. It was not until November 1998 when a group led by Dr. James Thomson developed a technique to isolate and grow embryonic stem cells from human blastocysts.[3]
Induced pluripotent stem cells were first produced in 2006 from mouse cells, and in 2007 from human cells, by Shinya Yamanaka at Kyoto University. The discovery was a vital advancement in stem cell research, because it allowed researchers to obtain pluripotent stem cells without the difficult legal, technical and ethical controversies that have long surrounded deriving cells from embryos. Yamanaka and his team reprogrammed adult mouse fibroblasts into iPSCs by introducing four reprogramming factors: Oct4, Sox2, c-Myc and Klf4. Subsequent work by James Thomson and colleagues replicated Yamanaka’s success with human cells and revealed additional factors, Nanog and Lin28, which facilitate the reprogramming process.[4]
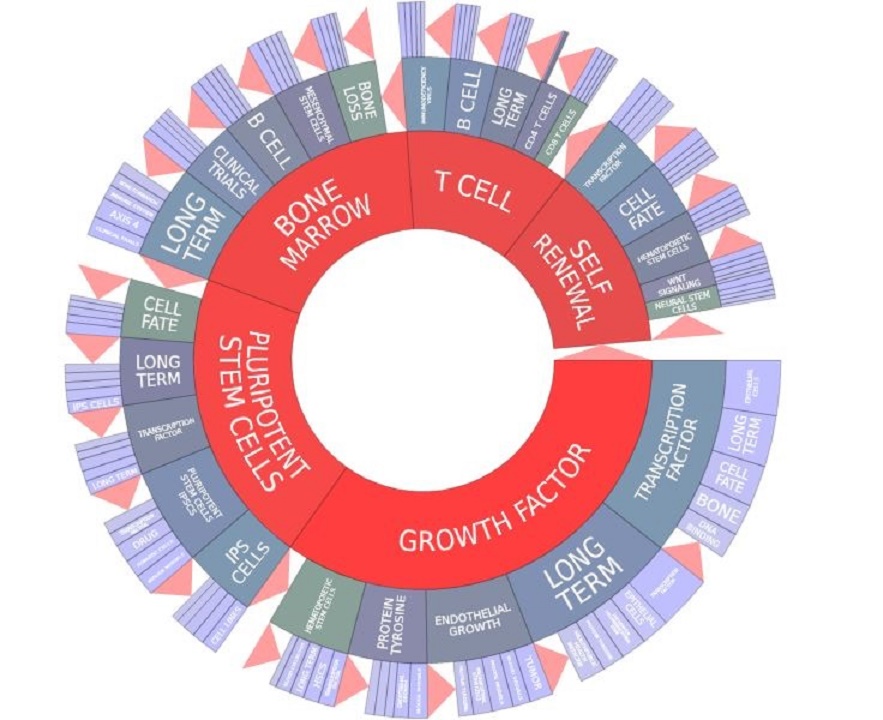
Currently, commonly investigated stem cell types include embryonic stem cells (ESCs), as well as adult stem cell types, such as mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), neural stem cells (NSCs), and induced pluripotent stem cells (iPSCs). There are also other less common stem cell types, including dental pulp stem cells (DPSCs), adipose-derived stem cells (ADSCs), and a variety of perinatal stem cell types, including amniotic stem cells, placental stem cells, and umbilical cord blood and cord tissue stem cells. Cancer stem cells also exist, giving rise to clonal populations of cells that form tumors or disperse within the body. Each of these stem cell types represents unique opportunities for research product development.
Furthermore, literature concerning the biology, characteristics, and applications of stem cells has flourished over the past few decades.
In full-year 2015, there were 25,613 stem cell scientific publications released, and an all-time search returns 259,218 stem cell scientific publications.[5]
Importantly, mesenchymal stem cells (MSCs) have greatly expanded in popularity of research, surpassing hematopoietic stem cells in 2015 for the largest number of stem cell scientific publications for the first time in history. Through 2014, hematopoietic stem cell research had always dominated other stem cell types within the scientific literature. However, there are more patents for embryonic stem cells (ESCs) than for any other stem cell type.
Geographically, the vast majority of stem cell clinical trials are being conducted within the United States and Europe.
Specifically, 2,792 (55%) out of 5,111 stem cell clinical studies occurring worldwide are being hosted within the United States. 1169 more are occurring within Europe (22%). Therefore, the rest of the world is contributing only 1,150 (23%) of all stem cell clinical trials worldwide.[6]
Furthermore, stem cells are increasingly being explored for use in 3D printing and biofabrication applications.
In 2015, RoosterBio and CELLINK signed a deal to use RoosterBio’s mesenchymal stem cells (MSCs) in “thaw and use” ink developed by CELLINK.[7] Additional announcements about the use of stem cells in 3D printing applications have also been surfacing, with Scottish researchers announcing the successful use induced pluripotent stem cells (iPSCs) in 3D printing without “adversely affecting their function” in October 2015 [8]. Shortly thereafter, in November 2015, a team of scientists from Tsingua University in China and Drexel University in Philadelphia released an article in the journal Biofabrication that introduced a “novel technique for printing a grid-like 3D structure laden with [embryonic] stem cells.”[9]
Clearly, the use of stem cells for 3D printing applications is an area of stem cell research that is expanding, because the technologies necessary to support 3D printing of stem cells were not readily available until recently. Therefore, 3D printing represents an interesting area for stem cell research product development. In particular, companies that can cost-effectively produce large quantities of stem cells will be strategically positioned to provide stem cell populations for integration in 3D printing inks.
Companies that are well-positioned in this area include Cellular Dynamics International – a global leader in the production of induced pluripotent stem cells; Cynata Therapeutics – an innovative company that can mass-produce mesenchymal stem cells using iPSCs from a single donor; Accellta – a company that specializes in growing stem cells extremely quickly and cost-effectively using bioreactors; RoosterBio – a company that uses disruptive technology to cost-effectively produce large volumes of MSCs, among others.
Currently, the stem cell research products market is dominated by a few key competitors. These companies have utilized acquisition strategies, as well as collaboration opportunities, to become industry behemoths.
The largest and most dominant players in the stem cell research products marketplace include:
- Thermo Fisher Scientific (Includes Invitrogen and Life Technologies Brands)
- BD Biosciences
- Miltenyi Biotec
- Merck KGaA (Includes Sigma-Aldrich Brand and EMD Millipore Brands)
- STEMCELL Technologies
- Lonza Group
- Clontech, a Takara Bio Company
- GE Healthcare Life Sciences
Cellular Dynamics International (CDI), now a FUJIFILM Holdings company, is also a major player in the stem cell research products marketplace, but it has chosen to exclusively focus on induced pluripotent stem cell (iPSC) products, instead of morphing into a broad-spectrum provider of stem cell products, like the other companies above. Corning is also a big company involved within the stem cell research products marketplace, but it specializes exclusively in labware and cell culture surfaces. It is also worth noting that Corning also has a partnership with STEMCELL Technologies and the Wisconsin Alumni Research Foundation (WARF) to promote its Corning® Matrigel® Matrix for use with mTeSR®1 medium, a product sold by STEMCELL Technologies under license from the WiCell Research Institute.[10]
In summary, the possibilities arising from stem cell characteristics have resulted in great commercial interest. Potential applications range from the use of stem cells in reversal and treatment of disease, to targeted cell therapy, tissue regeneration, pharmacological testing on cell-specific tissues, toxicology screening, and more. A large and growing stem cell research products market has emerged to facilitate research resulting from interest in these potential far-ranging applications.
Want to be better informed than your competition? Get future stem cell industry updates.
About Us
BioInformant is the only research firm to serve the stem cell sector since it emerged. BioInformant research has been cited by major news outlets that include the Wall Street Journal, Nature Biotechnology, CBS News, Medical Ethics, and the Center for BioNetworking. Serving Fortune 500 leaders that include GE Healthcare, Pfizer, Goldman Sachs, and Becton Dickinson, BioInformant is your global leader in stem cell industry data.
For more information on this market area, view “Stem Cell Research Products – Opportunities, Tools, and Technologies.”
Footnotes:
[1] Evans M, Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292(5819): 154–156.
[2] Martin G. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 1981; 78(12): 7634–7638.
[3] Thomson J, Itskovitz-Eldor J, Shapiro S, Waknitz M, Swiergiel J, Marshall V, Jones J. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282(5391): 1145–1147.
[4] Yu, J et al (2007). Science 318, 1917–1920.
[5] Ncbi.nlm.nih.gov,. “Home – Pubmed – NCBI”. N.p., 2016. Web. 25 Jan. 2016.
[6] ClinicalTrials.gov is a comprehensive resource for clinical trial data available at www.clinicaltrials.gov. Search Criteria: [“Stem Cell” OR “Stem Cells”]. Search Executed Jan 24, 2016.
[7] Product, RoosterBio. “Roosterbio Inc. Launches Industry’S First Ready-To-Print Stem Cell Product”. PRWeb. N.p., 2016. Web. 25 Jan. 2016.
[8] Millsaps, Bridget. “Scottish Researchers Are 3D Printing Extremely Delicate Stem Cells”. 3DPrint.com. N.p., 2015. Web. 25 Jan. 2016.
[9] Dean, Signe. “Scientists Have Found A Way To 3D-Print Embryonic Stem Cell ‘Building Blocks'”. ScienceAlert. N.p., 2016. Web. 25 Jan. 2016.
[10] Corning Product Flyer for “Corning® Matrigel® Matrix.” Available at: https://www.corning.com/media/worldwide/cls/documents/CLS-DL-CC-039.pdf . Web. 25 Jan. 2016.