Historical Number of Mesenchymal Stem Cell Clinical Trials (IFCB)
In 2014, the International Federation for Cell Biology (IFCB) conducted an analysis of mesenchymal stem cell clinical trials, identifying that 313 clinical trials involved mesenchymal stem cells (MSCs). The majority of these clinical trials (41.7%) were in Phase I/II. Another 23.7% of these studies were in Phase II. However, only 4.2% of these clinical trials had progressed to Phase III. As such, it is clear that the bulk of MSC clinical trials remain in the early stages of development.
Among the clinical trials that progressed through Phase III, one of the most promising stem cell therapies to emerge was Prochymal, an allogenic mesenchymal stem cell therapy derived from the bone marrow of adult donors and made by Osiris Therapeutics. Prochymal is produced by purifying MSCs from the bone marrow of donors, then culturing and packaging the cells, allowing up to 10,000 doses to be derived from a single donor.
In October 2014, regenerative medicine company Mesoblast Group acquired the culture-expanded mesenchymal stem cell business of Osiris Therapeutics, including the Prochymal product. Mesoblast and its Japanes licensee JCR Pharmaceuticals Co. Ltd. later re-branded Prochymal under the product name TEMCELL.
Prochymal/TEMCELL is used to address Graft vs. Host Disease (GvHD), a condition which often occurs during bone marrow transplantation, because the immune cells present in the transplant view the cells in the patient as foreign and start attacking the patient’s own cells and tissues.
It was the first stem cell therapy approved in Canada, as well as the first therapy approved within Canada to treat acute GvHD, making it a groundbreaking stem cell therapy product within the country. Within the United States, the FDA has granted Prochymal “Fast Track” review status. Therefore, Prochymal is also pioneering the way for MSC therapies to move toward new clinical applications in the U.S.
A 2014 breakdown of IFCB identified MSC clinical trials, by phase, is shown below.
FIGURE 4.4: Percentage of Mesenchymal Stem Cell (MSC) Clinical Trials by Different Phases
Terumo BCT Visualization of Mesenchymal Stem Cell (MSC) Clinical Trials
Additionally, there is also an absolutely outstanding data visualization for MSC clinical trials published by Terumo BCT, a global leader in blood component, therapeutic apheresis and cellular technologies.
Their data is from April 16, 2015 and cites “more than 300 clinical trials in various stages of evaluation.”
It is an interactive tool that gives industry professionals the power to sort and view the population of MSC clinical trials in various configurations, including by recruitment status, year, allogeneic vs. autologous, and by funding source.
It is available at: https://www.terumobct.com/assets/Data-Vis/MSC-Clinical-Trials/
ClinicalTrials.gov Analysis of Mesenchymal Stem Cell Clinical Trials (July 2015)
Most importantly, we have performed our own current (July 15, 2015), search of the ClinicalTrials.gov database and have returned 493 studies that contain the search phrase “mesenchymal stem cells.”
The same number of studies (493) are also returned when the search criteria is improved to include multiple relevant search terms (“mesenchymal stem cells” or “mesenchymal progenitor cells” or “multipotent mesenchymal stromal cells”).
While the ClinicalTrials.gov database is a U.S. based registry, it identifies itself as a “registry and results database of publicly and privately supported clinical studies of human participants conducted around the world.” As such, it is a fairly comprehensive, but not a complete database of registered clinical trials.
However, it is an outstanding resource to use for ascertaining mesenchymal stem cell industry trends, because it contains approximately three-quarters of registered cell therapy trials worldwide.
Other excellent registries that can be used to further bolster search results for mesenchymal stem cell clinical trials on a global basis include the European Union Clinical Trials Register and the Chinese Clinical Trial Registry (ChiCTR), among others.
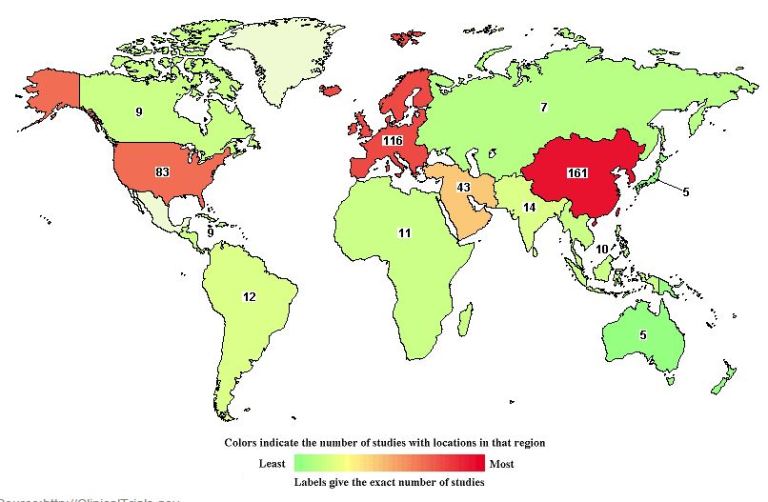
1. Geographical Assessment of MSC Clinical Trial Activity
When these 493 MSC clinical trials registered on the ClinicalTrials.gov database are assessed geographically, the following is found:
Clearly, the following regions are current hot-spots for MSC clinical trial activity:
- East Asia
- Europe
- United States
The Middle East is a second tier region for MSC clinical trial activity, trailing substantially behind the leading regions, but notably ahead of the rest of the world, at 43 registered studies.
2. Types of Sponsoring/Collaborating Organizations Supporting MSC Clinical Trial Activity
Also of interest to this analysis is a consideration of the types of “Sponsoring/Collaborating” organizations that are driving forward current MSC clinical trials. Again using the 493 results derived from the ClinicalTrials.gov database, a breakdown is shown below:
Currently, the top 3 types of sponsors/collaborators supporting MSC clinical trial activity are:
- Industry
- University/Organization
- Government, excluding U.S. Federal
3. Industry Participants Supporting MSC Clinical Trial Activity
Furthermore, because BioInformant is a research firm dedicated to serving stem cell industry executives and investors, the segment above that is of greatest interest to our audience is the “Industry” segment.
Therefore, a complete list of industry participants that are sponsoring/collaborating on MSC clinical trials registered in the ClinicalTrials.gov database is shown below, accompanied by the number of studies supported by each.
- Academy Military Medical Science, China 5 studies
- Alliancells Bioscience Corporation Limited 2 studies
- AlloCure Inc. 1 study
- AlloSource 1 study
- Anterogen Co., Ltd. 4 studies
- Apceth GmbH & Co. KG 1 study
- ArBlast Co.,Ltd. 1 study
- Bone Therapeutics S.A 1 study
- Brainstorm-Cell Therapeutics 1 study
- Bukwang Pharmaceutical 1 study
- CHABiotech CO., Ltd 1 study
- CardioCell LLC 2 studies
- CellMed AG, a subsidiary of BTG plc. 1 study
- Cellerix 2 studies
- Cellonis Biotechnology Co. Ltd. 3 studies
- Cellular Biomedicine Group Ltd. 2 studies
- Cryopraxis Criobiologia Ltda. 1 study
- Cytopeutics Pte. Ltd. 1 study
- Dong-A ST Co., Ltd. 1 study
- EMO Biomedicine Corporation 2 studies
- Genesis Limited 1 study
- Genexine, Inc. 1 study
- HomeoTherapy Co., Ltd 1 study
- Ivy Institute of Stem Cells Co. Ltd 4 studies
- K-Stemcell Co Ltd 8 studies
- Kang Stem Biotech Co., Ltd. 1 study
- Kasiak Research Pvt. Ltd. 2 studies
- Medipost America Inc. 1 study
- Medipost Co Ltd. 14 studies
- Medistem Inc. 1 study
- Mesoblast International Sàrl 15 studies
- Mesoblast, Ltd. 15 studies
- Orthofix Inc. 1 study
- Osiris Therapeutics 2 studies
- Pharmicell Co., Ltd. 6 studies
- Red de Terapia Celular 10 studies
- S-Evans Biosciences Co.,Ltd. 2 studies
- Salvat 1 study
- Shenzhen Beike Bio-Technology Co., Ltd. 7 studies
- Shenzhen Hornetcorn Bio-technology Company, LTD 2 studies
- Stemedica Cell Technologies, Inc. 3 studies
- Stempeutics Research Pvt Ltd 7 studies
- TCA Cellular Therapy 2 studies
- TFS Trial Form Support 1 study
- Taiwan Bio Therapeutics Co., Ltd. 1 study
- Teva Pharmaceutical Industries 1 study
- The EMMES Corporation 6 studies
- Thoratec Corporation 1 study
- TiGenix S.A.U. 2 studies
- Translational Biosciences 7 studies
Clearly, the following four companies are funding the greatest number of MSC clinical trials at this time:
- Mesoblast Ltd.
- Mesoblast International Sarl
- Medipost Co.
- Red de Terapia Celular
These companies are highlighted in the list above to emphasize their importance.
Summary of Findings for MSC Clinical Trials
The analysis above presents confirmatory findings from several different sources. The reason for including multiple sources is that a single data set is best suited to identifying unusual activity, while multiple data sets are best suited for confirming industry trends. Indeed, important industry trends will nearly always repeat and reappear when different data sets are considered. (Note: For more on the importance of utilizing high quality research inputs, read “Does Your Stem Cell Market Intelligence Provider Use Futuristic Techniques?”)
What is clear from each of these distinct sources is that MSC clinical trials are becoming substantial in number, growing year-over-year, and geographically clustered within specific regions of the world. Furthermore, new MSC therapy products will continue to enter the marketplace, as large regenerative companies that include Mesoblast, Medipost, and Red de Terapia Celular, choose to fund product development efforts involving the cell type.
About BioInformant
BioInformant is the first and only research firm to specialize in the stem cell industry. Our management team comes from a BioInformatics background – the science of collecting and analyzing complex genetic codes – and applies these techniques to the field of market research. BioInformant has been cited by prominent news outlets that include the Wall Street Journal, Nature Biotechnology, Xconomy, and Vogue Magazine.
To learn more, view the “Mesenchymal Stem Cells – Advances & Applications.”