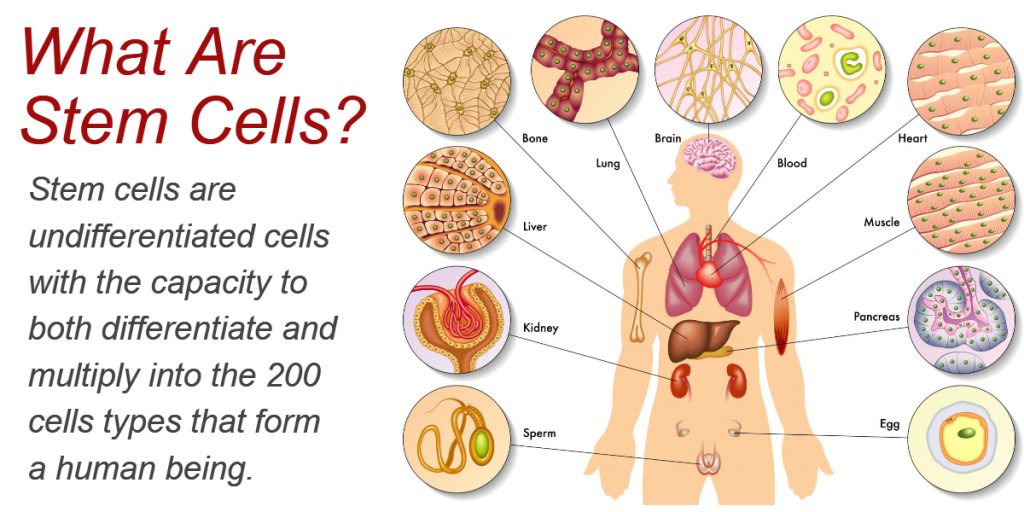
Stem cells are undifferentiated cells that have the capacity to both differentiate and multiply into the 200 cells types that form a human being. In total, the human body is composed of an estimated 30 trillion cells, making stem cells extremely important to human development. Stem cells are also found in plants and animals.
The use of stem cells to treat human disease is being investigated within scientific research, as well as thousands of clinical trials worldwide. These cells are bringing hope to many people worldwide, including patients, researchers, and clinicians.
For the bulk of human history, a serious injury or long-lasting disease meant death. Whether the problem was infection, degeneration or failure of a vital system, healers were at a loss for what to do. The problem? They couldn’t figure out how to replace systems that were damaged. This is where stem cells have the potential to create a paradigm shift.
What exactly are stem cells and how has are they changing the way that diseases and injuries are treated?
What You Need To Know About Stem Cells
In this article:
- How Have Stem Cells Changed Treatment For Degenerative Diseases
- How Do Cells Work?
- Why Don’t All Cells Reproduce Forever?
- How Do Stem Cells Help?
- A Brief History of Stem Cell Research Around the World
- What Types of Stem Cells Exist?
- How Are Stem Cells Used in Medicine?
- What Diseases Can Stem Cells Possible Help Treat?
- How Do Physicians Harvest Stem Cells?
- How Do Scientists Force Stem Cell Development Along Certain Lines?
- What Are the Risks Associated with Stem Cell Therapy?
- What Does Stem Cell Therapy Cost?
- What Other Roadblocks Exist?
How Have Stem Cells Changed Treatment For Degenerative Diseases
Regenerative medicine, which relies on the near-magic of human stem cells, has come far in the last two decades. For medical problems that once seemed past all hope – such as leukemia and other types of cancer, degenerative diseases like osteoarthritis, or traumatic injury to the body – humanity now has a range of solutions based on actually regrowing injured or damage systems.
While stem cells have played a prominent role in scientific research and news for years, many people still don’t understand exactly what they are or how they work. Worse, many people believe outright that they’re an unacceptable approach to medical needs. That’s because the early history of stem cells relied heavily on controversial embryonic sources.
Luckily, that’s no longer the case. The ready availability of adult stem cells obviates the need to obtain them from fetuses. Also, the advanced pace of regenerative medicine has improved our ability to fight injury and disease.
Anyone considering a stem cell treatment, or simply wishing to know more about this scientific breakthrough, should read on.
How Do Cells Work?
The human body comprises trillions of cells. When like cells work together, these are known as tissues. We have many types of tissue, such as nervous, cardiac, liver and so much more.
Cells are complex structures that have dedicated purposes, depending on their location and function in the body. In other words, the inner and outer structure of each cell varies with the job it does. However, they share similarities across tissue types, including an outer membrane, inner organelles (the cell’s own “organs”) and a nucleus containing our genetic code or DNA.
By dividing constantly, cells are able to maintain the functionality of muscles, organs, tissues, and blood. Over time, the older cells die off and get recycled by the body, while younger cells take their place. The result is a cycle of birth that mimics life itself. It keeps a human body going for as much as a hundred years – and maybe more.
Unfortunately, while cells are nothing short of miraculous in their machinery, most cell divisions can’t keep going forever.
Why Don’t All Cells Reproduce Forever?
Some parts of the body do produce more stem cells on their own. For instance, bone marrow is capable of producing stem cells that differentiate into various types of blood cells. For that reason, most people do not need to worry about their blood becoming compromised over time, except in cases where the cells themselves are defective (ex. sickle-cell anemia), or cases where the bone marrow itself suffers damage (ex. leukemia). Normally, though, if you have blood drawn, or even if you lose a lot of it to injury, you can make more.
Other parts of the body are not so lucky, however. We come into this world with a limited number of some types of cells. Other cell types can proliferate, but to a limited extent. When damage occurs to the heart or brain, if it is severe enough, there is usually no saving the victim.
The same is true for cartilage cells. Degenerative conditions such as osteoarthritis, in which cartilage in the joints breaks down over time, eventually result in a painful grinding of bone on bone. In some cases, surgery or over-the-counter medicine can help. In others, steroid injections may provide relief. But, few approaches (if any) can help the cartilage to grow back.
Then there is the fact that cells simply get “old.” After a certain number of divisions, the cells can start to have problems. That’s because the ends of each chromosome (strands of DNA all coiled up into compact shapes), start to unravel. These sites – called telomeres – degrade over time, resulting in worse and worse copies of your genetic information. These “senescent cells” just aren’t good anymore.
The result? Aging. Systems break down. Cells can’t replace themselves as effectively. People die.
How Do Stem Cells Help?
Stem cells solve this problem of finite cell division, as well as the problem of traumatic injury to parts of the body that cannot repair themselves.
Unlike regular cells, which have a dedicated role, stem cells can turn into many types of cell. So, for instance, a muscle cell could never fill in for a blood cell or heart cell. That’s not what it’s made for. However, a stem cell could see a need in the body, head to that location (called “honing”) and become the necessary cell type through a guided transformation involving chemicals, growth factors and other complex determinants.
In addition, stem cells are the foundation of development in complex plants and animals. The embryo, or extremely early life stage, contains stem cells. As development progresses, these stem cells turn into the different cell types needed to create a fully functioning human, cow, or tree. They turn into the tissue-specific cells discussed above.
Some types of stem cells (discussed below) are only present in the earliest stages of development, while other types remain throughout the remainder of fetal development (in mammals, anyway). Still, others remain present in the body throughout an adult’s lifetime.
Once scientists discovered this, the question became: How could medicine leverage these cells to replace structures that ordinarily couldn’t get replaced? How could they effectively turn back the clock on injury, disease or aging?
A Brief History of Stem Cell Research
Researchers have known since the early 1900s that some cells had the ability to generate blood cells in the body. In the late 70s, scientists discovered stem cells in human umbilical cord blood, and shortly thereafter, created the first successful in vitro (outside the body) stem cells. Further breakthroughs included successful stem cell lines created from a hamster in 1988, primate stem cells in 1995, a cloned lamb in 1997, and embryonic stem cell lines in 1998.
This kicked off a serious disagreement between those who believe it is wrong to use human embryos for scientific research and those who think embryos are a critical tool in the development of scientific research. (BioInformant takes no stand on this issue.) For years, stem cell research got mired at this impasse, research put even further behind with George W. Bush’s executive order banning stem cell experimentation in 2001.
Five years later, according to research reported in The New York Times, Japanese scientists found a way around this. Kyoto University scientist Shinya Yamanaka and graduate student Kazutoshi Takahashi discovered a means of reversing cell development, turning adult cells back into pluripotent stem cells. By obviating the need to obtain stem cells from embryos, these two researchers revitalized the field and paved the way for regenerative medicine to take off in true fashion.
The first successful stem cell transplant occurred in 1969, using donor cells from a healthy person to treat severe combined immunodeficiency in two sibling patients. Since then, stem cell transplants and stem cell therapy have proven viable in a huge range of cases.
Before discussing those, however, it is important to understand what types of stem cells exist, to better understand their use in medicine.
What Types of Stem Cells Exist?
A range of stem cells exist. The first distinction is between adult and embryonic stem cells, while the second main distinction is between the level of capability stem cells possess to turn into other types of cells. Note the overlap between the two categories.
Embryonic Stem Cells
When two gametes unite to form an embryo, new life is initiated. After 3 to five days, the embryo becomes a blastocyst composed of roughly 150 cells. During this time, embryonic stem cells start to form. Present at the earliest stages of life, embryonic stem cells can form any of the more than 200 cell types present in the human body.
Quickly, embryonic stem cells start to specialize and lose their ability to turn into any type of cell.
Adult Stem Cells
Adult stem cells are present in the human body after birth, during childhood, and throughout the adult lifespan. They exist in a number of places, including within the teeth, liver, brain, skeletal muscle, gut, ovarian epithelium, testis, heart, and a few other places. However, the most common places from which to harvest those cells today include the bone marrow, adipose tissue (fat cells), and peripheral blood.
Tissue-Specific (Multipotent) Stem Cells
Adult stem cells are tissue-specific, meaning that they can become a limited number of cell types. An example of tissue-specific stem cells includes the mesenchymal stem cells (MSCs), which can develop into many of the cells associated with the skeletal system, such as bone cells, cartilage cells, muscle cells, and fat cells. Similarly, hematopoietic stem cells (HSCs) can give rise to a wide range of blood cells, including white blood cells, platelets, red blood cells and more.
Tissue-specific stem cells are multipotent stem cells, because they can turn into many different, but not all, types of cells.
Pluripotent Stem Cells
Pluripotent stem cells are so named because they are able to turn into any cell in the body. These are found only in the earliest stages of embryonic development. In 2006, scientists also discovered a way to “induce” pluripotency, as described below in the section on induced pluripotent stem cells (iPSCs).
Totipotent Stem Cells
Totipotent stem cells have all the same abilities as pluripotent stem cells, in that they can turn into the building blocks of any tissue in the body. However, they also possess the ability to grow into cells of the embryo and developing fetus, which no other stem cell type can do.
Induced Pluripotent Stem Cells (iPS Cells)
This stem cell type, more than any other, has the medical community excited. Induced pluripotent stem cells (iPSCs or iPS cells) are adult stem cells that scientists have coaxed back to an earlier stage of pluripotency, which is the ability to turn into any cell within the human body.
The discovery of iPS cells is widely credited to Dr. Shinya Yamanaka of Kyoto University.
In August 2006, Dr. Yamanaka and his team generated iPS cells from adult mouse fibroblasts for the first time. By 2007, iPS cells were concurrently generated from human cells by Dr. Yamanaka’s team, as well as by a research team at the University of Wisconsin led by James Thomson. Thomson’s team published their findings in Science in a 2007 article titled “Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells.”
In 2012, Shinya Yamanaka was awarded the Nobel Prize in Physiology or Medicine for the discovery that mature cells can be reprogrammed to become pluripotent.
These cells could potentially give physicians the tools needed to replace parts of the human body, without having to harvest these cells from an embryonic state.Induced pluripotent stem cells are still in the early stages of clinical development, but research is progressing.
In a world first, Australian-based Cynata Therapeutics launched the world’s first clinical trial of an iPS cell derived therapeutic product, treating its first patient in May 2017. This iPS cell derived product (CYP-001) is being explored for its ability to treat a devastating disease called Graft vs Host Disease (GvHD).
There are also a number of physician-led clinical studies underway in Japan that are exploring the use of iPSC-derived cell types for the treatment of macular degeneration (eye disease), Parkinson’s disease, and heart disease, as well as the use of iPSCs to create an unlimited supply of human platelets.
How Are Stem Cells Used in Medicine?
Stem cells are used in a variety of ways in medicine. For instance, when a person has a blood or bone marrow disorder, they may receive a stem cell transplant. If they have lost the ability to manufacture their own new marrow or blood cells, then replenishing their stem cells will renew that function.
Stem cells also offer an unprecedented opportunity to restore damaged nerves and heart cells. Formerly, physicians were only able to manage conditions associated with such damage. That historically meant that heart disease, Alzheimer’s, brain damage and other catastrophic conditions were permanent. While the patient might still have years of life ahead of them, they typically couldn’t expect to regain any lost function.
Soon, however, scientists might have the opportunity to treat such conditions by injecting stem cells at the site of the problem. Along with growth factors and other compounds, the stem cells have the potential to react to their conditions in several different ways. Some types of stem cell, such as MSCs, can exert therapeutic effects by reducing inflammation, reducing fibrosis (scarring), and positively impacting the regulation of the human immune system. Other types of stem cells, such as iPSCs, may be able to repair tissue by replacing damaged cells.
It’s also possible that researchers could use stem cells to grow new organs. In the future, someone who needs a heart transplant may be able to receive a new, fully grown heart. We scientists haven’t developed this technology yet, many researchers are exploring the role of stem cells within these types of tissue engineering applications. By using a patient’s own stem cells to create it, it would also eliminate the risk of organ rejection or a dangerous immune response by the patient.
While technology such as this is still years away, hope may be on the horizon.
What Diseases Can Stem Cells Possible Help Treat?
These are just a few examples of the types of issues researchers are working to solve with stem cells. Theoretically, other potential breakthroughs that stem cells could facilitate in the near (or far) future include:
- Treating neurological diseases throughout the body and brain
- Replacing organs damaged or lost to disease or traumatic impact
- Responding to autoimmune diseases that attack the body
- Helping people who have trouble manufacturing new cells do so
- Treating degenerative diseases, such as back problems
- Managing all types of arthritis
- Treating cancer patients and replacing diseased areas of the body
How Do Physicians Harvest Stem Cells?
Typically, researchers harvest stem cells from peripheral blood, bone marrow, or fat tissue. Adult stem cells are present in other parts of the body, but they can be harder to access or require collection at a certain point in human development (for example, dental pulp stem cells). However, peripheral blood, bone marrow, and fat stores are ready sources of stem cells. Once researchers learn more about the use of inducing pluripotent stem cells within clinical applications, therapeutic options may further expand.
Depending on whose stem cells the patient is using, the harvesting process looks different.
Using Your Own Stem Cells
When a patient uses their own cells, those cells get harvested and isolated using a centrifuge or other device. Sometimes they get augmented in a lab over time to increase their number, after which they get injected back into the patient. Researchers refer to this as an autologous transplant, and essentially means that the procedure uses the patient’s own cells from start to finish.
Donor Cells
An allogeneic transplant, on the other than, is when a patient receives donor cells. This may occur because the patient’s cells are too compromised. For instance, they might have cancer, and using their own stem cells runs the risk of reintroducing that cancer to the system they’re trying to save. In that case, healthy donor cells from someone else are the best bet. Donor cells may come from:
- A family member who offers blood, bone or fat samples
- Banked cord blood, found inside the umbilical cord of a newborn infant, donated by parents
- Banked stem cells from anonymous donors
While it is wonderful that people are willing to donate their stem cells, the chances of a match between two unknown parties are relatively small.
There is a third option for forward-thinking patients, who bank (store) umbilical cord blood stem cell from their newborn children. Those who banks healthy stem cells at birth will later have these cells available for use in the case of disease or injury. If used by the child from whom they were collected, the cells will be a perfect match and will prevent an immune response if used later in life.
How Do Scientists Direct Stem Cell Development?
The question of how scientists force stem cells to develop into a certain type of cells is an interesting one, and the mechanisms are not yet entirely clear. Before considering this process, it is important to note that the therapeutic capacity of stem cells are not limited to their ability to differentiate into specific tissue types.
Meaning, stem cells can also exert therapeutics effects through other mechanisms that do not require differentiation. For example, stem cells are widely known to exert therapeutic effects by modulating inflammation, reducing fibrosis (scarring), or mediating an improved immune response.
Also, the strategies used to differentiate stem cells into specific cell types will vary depending on whether a scientist is handling them within a laboratory setting (know as in vitro) versus introducing them into a human patient (known as in vivo). Understandably, scientists have more tools available to “force” differentiation when they are handling stem cells within a lab. It is substantially more difficult and complex to modulate stem cells once they are injected or infused into a human patient.
With that in mind, one of the techniques that medical doctors can use to direct stem cell function is to select how and where they are administered to a patient. For example, stem cells injected into a damaged heart will have a different effect than stem cells that are introduced intravenously into the blood stream. The type of stem cell chosen by the physician will also have major impact, because different types of stem cells behave differently.
Furthermore, scientists can often offer certain types of stimuli to stem cells to help them behave in a desired way. These stimuli can include growth factors (the chemicals that tell stem cells how to grow) and morphogenic factors (chemicals that instruct stem cells about which types of tissue to become), for example.
Stem cells also need structural support, which is where “scaffolding materials” come in. These are materials, sometimes organic and sometimes manufactured in a lab, onto which stem cells can grow to create new bodily tissues. Tissue engineering is still in its infancy, but has proven a key ingredient to inducing the growth of new organs and other tissue types.
While researchers still know a limited amount about what activates stem cell behavior and what causes them to become other cells, knowledge in this area is expanding.
What Are the Risks Associated with Stem Cell Therapy?
There are a number of risks associated with stem cell therapy.
First and foremost, the risk of graft-versus-host disease attends any allogeneic stem cell transplant – one that uses donor cells instead of the patient’s own cells. In this case, the donor cells may, upon entering the host body, initiate an immune response against the host. They attack host cells as though they were a foreign invader in the donor’s own body. This can cause diarrhea, rash, eye irritation, and, if it continues long enough, even death.
Of course, it’s also possible that the host will reject the transplant as well, which can also cause sickness and if the transplant doesn’t take, death.
Other risks are the same as those that attend any procedure. Infection is always a hazard. While antibiotics usually take care of the bacterial infections that might develop at the site of incision, there’s no guarantee that infection won’t develop anyway. Similarly, it is possible for a patient to die under anesthesia no matter the procedure, especially if they are older or in delicate health.
One of the biggest risks of stem cell transplant, however, is the potential that cells may grow uncontrollably. Uncontrolled cells are basically the definition of cancer, which obviously presents a serious problem. Scientists need more research to determine what will reduce or eliminate these risks to the greatest degree possible.
What Does Stem Cell Therapy Cost?
One of the biggest remaining obstacles in stem cell research is the cost associated with treatments. Currently, most health insurance companies do not pay for stem cell treatments, which leaves patients with large out of pocket expenses to cover. While some people have the funds to do this, many do not.
For instance, stem cell treatment for a knee may cost anywhere between $5,000 and $20,000. Depending on the severity of the knee’s cartilage breakdown, the age of the patient, the number of procedures required and the clinic, these costs range wildly.
This also depends on the state or country in which the patient lives, what the regulations are there, where they’re willing to travel, how much travel and lodging costs, how long the procedure takes, and so forth. Other orthopedic conditions may cost less.
Stem cell treatment for hair, on the other hand, shows great promise for filling in bald patches and thickening existing hair. It too is expensive, however, costing between $3,000 and $10,000, depending on the number of treatments needed and the amount to fill in.
There are a few procedures, however, typically covered by insurance. One of these is stem cell bone marrow transplants. This technology has proven effective for leukemia and other bone/blood disorders. Because it costs between $350,000 and $800,000, due to the complexity of the procedure and the length of time it takes to complete, it wouldn’t prove feasible without insurance.
What are FDA Approved Stem Cell Treatments?
Beyond bone marrow transplants, the only stem cell treatments approved by the U.S. FDA are ones that use umbilical cord blood to treat various cancers. Of the 16 FDA-approved cell and gene therapy
Approved cord blood derivatives include ALLOCORD, CLEVECORD, HEMACORD, and Ducord, as well as four hematopoietic progenitor cell (HPC) products developed by Clinimmune Labs, MD Anderson Cord Blood Bank, LifeSouth Community Blood Centers, and Bloodworks, respectively.
As stated by the FDA, “The only stem cell-based products that are FDA-approved for use in the U.S. consist of blood-forming stem cells (hematopoietic progenitor cells) derived from cord blood. These products are approved for limited use in patients with disorders that affect the body system that is involved in the production of blood.”
What Other Roadblocks Exist?
Although there are now over 6,300 clinical trials worldwide involving stem cells, the true power of stem cells lies in the future. Regrowing organs or limbs or brain matter is far down the road, but researchers work toward it every day.
In the end, stem cells promise medical advancements that humanity couldn’t have dreamed up only a few decades ago.
Looking for more information about stem cells? BioInformant is the world’s largest stem cell industry news site, with research cited by The Wall Street Journal, Xconomy, andVogue Magazine. Sign up today to stay informed.
Seeking a Stem Cell Treatment?
As the world’s largest publisher of stem cell industry news, BioInformant understandably cannot provide clinical treatments or advice. For this reason, please contact GIOSTAR with your medical questions. GIOSTAR is a global stem cell company that has treated a large number of patients in the U.S. and worldwide.
You can reach them at this link to schedule a consultation or ask them your questions.
If you found this blog valuable, subscribe to BioInformant’s stem cell industry updates.