In this interview, we discuss Minerva’s technology platform, product portfolio, IP strategy, and strategic goals. We also explore the significance of the company’s AlphaSTEM™ Culture System that is a simple method for inducing the naïve state in human stem cells. Enjoy.
Minerva Biotechnologies, Interview with Dr. Bamdad
Cade Hildreth: What is your background how did you decide to found Minerva?
Dr. Cynthia Bamdad: I have a B.S. in physics from Northeastern University and a Ph.D. in Biophysics from Harvard. In the mid 1990’s while at Harvard, I invented an electronic DNA detector. It was licensed to a California startup, Clinical Micro Sensors, where as Chief Scientific Officer I developed the electronic DNA detector into a commercial product.
That technology is now an FDA approved diagnostic device marketed by Genmark. I thought that a variation on that technology could be used to study growth factor receptors – particularly to study mechanisms of cancer growth and survival. My mother died young of breast cancer, so I have a special interest in finding a cure.
Cade Hildreth: I know your company works in the areas of stem cells and cancer cells. How do you view the relationship between stem cells and cancer cells?
At the center of this self-replication is MUC1, which was known for over two decades to be aberrantly expressed on over 75% of cancerous tissues, however, no one had been able to show a functional link between MUC1 and cancer. We discovered that on cancer cells, and importantly also on stem cells, MUC1 is cleaved and the bulk of the extracellular domain is shed from the cell surface.
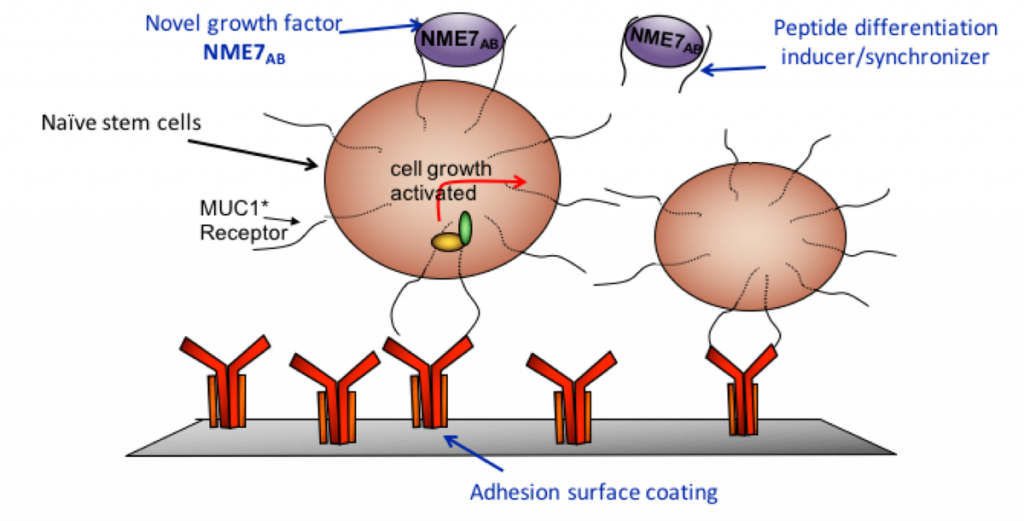
The remaining portion that we call MUC1* (pronounced muk 1 star) is a growth factor receptor that is activated by NME1 or NME7AB binding to and dimerizing its extracellular domain. NME1 and NME7AB are only secreted from stem cells and cancer cells. NME7AB expression and secretion should stop after the first few days of a developing embryo, but we find it is turned on again in cancers.
One of the mysteries was if stem cells and cancer cells grow by the same mechanism, then how do stem cells limit their self-replication but cancer cells do not? Over the last 12 years, Minerva elucidated the molecular mechanism behind how stem cells limit their growth and how cancer cells override the natural shut off switch to keep growing forever. The mechanism is reminiscent of how the most primitive eukaryotes, phage lambda, regulate their growth – through changes in the multimerization state of a key protein. It makes sense, right?
The most primitive mechanism of gene regulation that lambda uses to decide growth or dormancy is essentially the same mechanism that our most primitive cells – stem cells – use to decide whether to grow pluripotently or differentiate. Both are key fate decisions. Cancer cells have just hijacked that primitive growth mechanism and override the shut off switch.
So that brings us to where we are today. First, Minerva has a novel stem cell media that puts stem cells into a stable naïve state and secondly, we are in late stage development of a cancer immunotherapy that inhibits the stem-like mechanism that is the essence of cancer.
Cade Hildreth: What are naïve state stem cells and what are their advantages?
True naïve stem cells are free of cell fate decision marks, making it easier to direct their differentiation to specific cell types. As we learn more about the naïve state, it is becoming clear that there are even more fundamental differences between naïve and primed state stem cells that significantly limit the ability of primed state stem cells to differentiate into certain cell types.
Cade Hildreth: Are there others making naïve stem cell media and how does Minerva’s compare?
Dr. Cynthia Bamdad: Yes, others have made naïve media. But unlike Minerva’s media, these are more or less magic potions, containing concoctions of growth factors and biochemical inhibitors that somehow make stem cells behave better. We have no idea how some of those components affect stem cells and, indeed, many of the other naïve media induce karyotype instability.
In contrast, the Minerva media is a minimal serum-free media containing one active ingredient: NME7AB. It is the naturally occurring growth factor that is secreted by stem cells of the inner cell mass. In addition, Minerva’s naïve media increases the efficiency of iPSC generation by orders of magnitude and the resultant cells are in the naïve state.
Cade Hildreth: When did you launch your AlphaSTEM™ Culture System and what is the importance of this culture system?
Dr. Cynthia Bamdad: We launched in February 2017. The entire system includes the NME7AB containing naïve media, an adhesion surface and a synthetic peptide that breaks the interaction between NME7AB and MUC1* to induce differentiation and reduce the risk of teratoma formation. Our surface coating captures stem cells without introducing other growth factors or other biological signals that induce the primed state, even when used with naïve media. We have shown that popular stem cell growth surfaces such as MEFs, Matrigel, and Vitronectin all increase expression of primed state markers.
Cade Hildreth: What has been your intellectual property (IP) strategy and what IP rights have you secured?
Dr. Cynthia Bamdad: We have issued patents that protect our media, surfaces and differentiation-inducing peptide, as well as patents pending for some products that are in our pipeline.
Cade Hildreth: What are your 3-5 year goals for Minerva Biotechnologies?
Dr. Cynthia Bamdad: One of our near-term goals is to enter into one or more partnership agreements with companies developing stem cell based therapeutics. There are currently several barriers to the development of stem cell therapeutics for widespread use, which include the difficulty of:
- Generating high quality iPS cells from multiple donors representative of a diverse population
- Economically and consistently growing large numbers of high quality stem cells
- Obtaining stem cell derived cells that are functional equivalents of naturally occurring cells
- Obtaining high yields of the desired cell type
- Eliminating the risk of teratoma formation
The Minerva technology solves all of these problems.
We are also very motivated to work with governments or government agencies in programs to generate banks of iPS cells. For example Japan has a program to develop iPS cells from HLA homozygous donors. This approach enables entire populations to be treated with cells from just a few iPS cell lines. Minerva’s technology is complementary to the Yamanaka iPS technology; NME7AB continues the iPS reprogramming all the way back to the naïve state. We have also shown that the efficiency of iPS cell generation goes up by orders of magnitude when NME7AB is added to the core pluripotency factors.
On the cancer side, we will file an IND application for our lead cancer therapeutic, which is a cancer immunotherapy for solid tumor cancers, by the end of this year and enter into clinical trials in early 2018. Cancer immunotherapy is an exciting approach to curing cancer but so far has only been successful in treating blood cancers which are only 7% of all cancers. The other 93% are solid tumor cancers. Over 75% of solid tumor cancers express MUC1* which is a powerful growth factor receptor that drives cancer growth.
Incidentally MUC1* is the growth factor receptor that drives the growth of 100% of pluripotent stem cells. For a cancer immunotherapy against solid tumor cancers to be safe, you need to identify a target that looks different on cancer cells than it does on healthy cells. That’s what we’ve done. Making a cancer immunotherapy against solid tumor cancers that is effective is an unanticipated problem that we believe we have solved.
Cade Hildreth: How can people learn more about Minerva or get in touch with you?
Dr. Cynthia Bamdad: We are always interested in collaborating with innovative researchers in academia and industry, particularly those seeking therapeutic applications in regenerative medicine. We welcome contacts through our website www.minervabio.com, or directly to our business development team:
Matt Britz, mbritz@minervabio.com
John Rogers, jrogers@minervabio.com
James Lovgren, jlovgren@minervabio.com