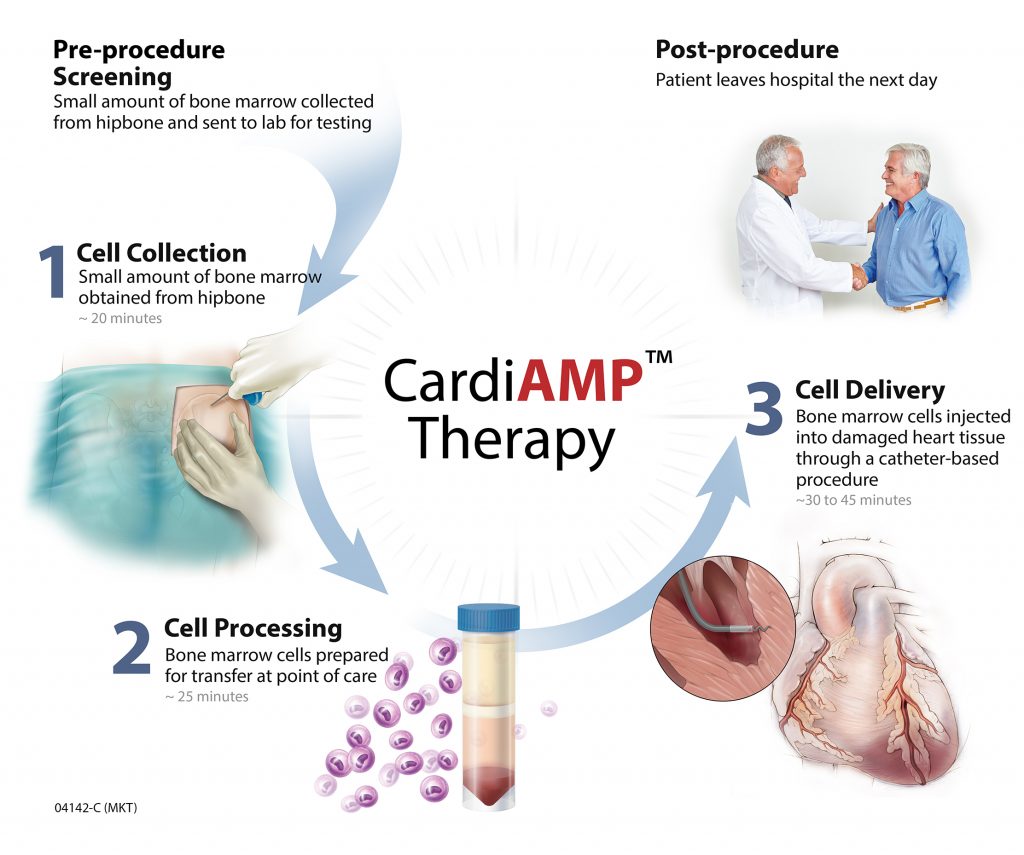
Circulation Research, the official journal of the American Heart Association, has highlighted a novel stem cell therapy approach developed by BioCardia. For those not familiar with BioCardia, the company specializes in developing regenerative biologic therapies to treat cardiovascular disease.
BioCardia has developed a stem cell technology that includes a novel method of administration, as well as a pre-procedure assay to achieve a comprehensive approach to heart failure. BioCardia’s CardiAMP and CardiALLO cell therapies are the Company’s biotherapeutic product candidates in clinical development.
In addition to providing Phase III 10-patient roll-incohort data for BioCardia’s CardiAMP device, the paper published by Circulation Research offers a comprehensive rationale of CardiAMP’s development as a treatment for heart failure.
Optimizing Patient Selection for Cardiac Regeneration
In the article entitled, “Not All Stem Cells Are Created Equal: The Case for Prospective Assessment of Stem Cell Potency in the CardiAMP Heart Failure Trial,” Peter Johnston, M.D. and his co-authors state: “Using a ‘personalized’ medicine approach (i.e. tailoring treatment to patient characteristics to optimize benefit), we propose to optimize cardiac cell therapy efficacy by prospectively selecting patients most likely to benefit using a cell potency assay to assess inherent regenerative capacity.”
While administration has long been a focal point by developers of stem cell technologies, there has been little focus on optimization of tissue and patient selection for cardiac regeneration.
In contrast, this paper provides rationale for use of BioCardia’s cell potency assay to:
- Screen for patients with heart failure most likely to respond to BioCardia’s CardiAMP therapy
- Address an aspect of variability seen in previous cardiac stem cell trials
Additionally, the publication highlights supportive, statistically significant, early data from the ongoing Phase III pivotal trial in patients with heart failure that is still enrolling.
BioCardia’s CardiAMP Heart Failure Trial
There are currently only three programs with approved pivotal randomized controlled trial (RCTs) in the adult restorative heart therapy space. These programs are BioCardia’s CardiAMP trials in Heart Failure & Chronic Myocardial Ischemia and Mesoblast’s Revascor program.
All three of these trials use cells derived from the bone marrow and delivered into the heart muscle as opposed to the coronary artery (previous methodology).
Additional details about BioCardia’s Phase III pivotal trial in patients with heart failure are below:
- BioCardia’s Phase II trial I Heart Failure met its primary endpoint with statistical significance and clinically meaningful improvement vs. placebo in the primary clinical endpoint used in the Phase III study
- The Phase III trial includes a pre-procedure assay that supports selection of patients most likely to respond to therapy
- The trial has CMS reimbursement
With BioCardia bringing together stem cell technology, devices and now diagnostics, it is on track to achieve a comprehensive approach to heart failure.
To learn more, view the company’s recent press release or watch the video below about the Helix Transendocardial Delivery System.
Up Next: Cardiac Stem Cell Therapies: The Next Revolution in Heart Failure Treatment