Numerous induced pluripotent stem cell (iPSC) derived therapeutics are now being studied in preclinical and clinical trials to investigate their potential to produce functional cells capable of replacing damaged or dysfunctional tissues.
It is important to note that the early clinical trials of iPSCs—and the majority of them today—do not involve the transplant of iPSCs into humans. Rather, they involve the creation and evaluation of iPSC lines for clinical purposes. Within these trials, iPSC lines are created from specific patient populations to determine if these cell lines could be a good model for a disease of interest.
iPSC Clinical Trials Involving Cellular Therapeutics
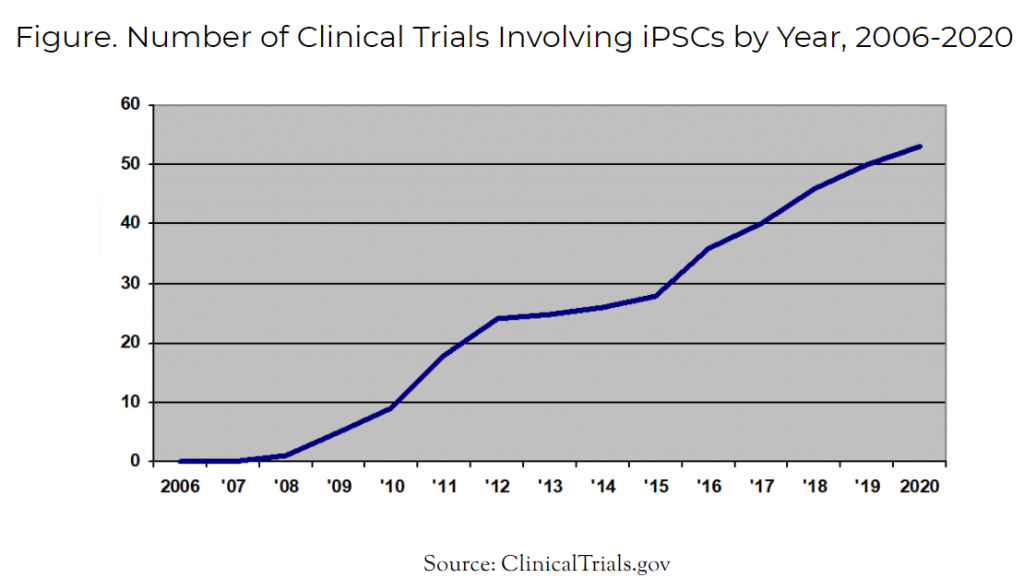
The first clinical trial using iPSCs for any purpose occurred in 2008. Today in 2021, the total number of iPSC clinical trials has risen to 54. Of these 54 trials, a select few are now administering iPSC-derived cell therapeutics to human patients.
These cell therapy trials include:
- CYP-001, an iPSC-derived MSC product by Cynata Therapeutics
- CYP-004, also an iPSC-derived MSC product by Cynata Therapeutics
- FT500, an iPSC-derived NK cell product by Fate Therapeutics
In 2016, Cynata Therapeutics received approval to launch the world’s first formal trial of an allogeneic iPSC-derived cell product (CYP-001) for the treatment of GvHD. CYP-001 is a iPSC-derived mesenchymal stem cell (MSC) product. In this historic trial, CYP-001 met all of its clinical endpoints and produced positive safety and efficacy data for the treatment of steroid-resistant acute GvHD.
Consequently, Cynata is now advancing its iPSC-derived MSCs into Phase 2 trials for the treatment COVID-19, GvHD and critical limb ischemia (CLI). It is also undertaking a massive Phase 3 trial that will utilize Cynata’s iPSC-derived MSC therapeutic, CYP-004, in 440 patients with osteoarthritis (OA).
Riding the momentum within the CAR-T field, Fate Therapeutics Inc is developing FT819, its off-the-shelf iPSC-derived CAR-T cell product candidate.
Figure. Number of Clinical Trials Involving iPSCs by Year, 2006-2020
iPSC Progress in Japan
Within Japan, there are also several physician-led studies underway investigating the use of iPSC-derived cellular products within human patients. While these are not formal clinical trials, they do involve the transplant of iPSC-derived cellular therapeutics into human patients.
The first of these occurred in 2013 when a Japanese woman with macular degeneration (vision loss) received a transplant of iPSC-based retinal cells developed from her own cells. While she did not experience improvement in her vision, the safety of the iPSC-derived cells was confirmed in this study. By 2017, five patients were treated with iPSC-derived retinal cells for macular degeneration and one patient developed an adverse reaction.
In a surgical procedure performed in October 2018, neurosurgeons from Kyoto University implanted 2.4 million cells into the brain of a patient with Parkinson’s disease. The iPSCs obtained from peripheral blood cells of a donor were reprogrammed into induced pluripotent stem cells (iPSCs) and then differentiated into dopaminergic precursor cells. The hypothesis is that these cells will boost dopamine levels and improve the patients’ symptoms.
Japanese researchers also launched a clinical study to evaluate the efficacy of iPSCs in heart disease. In 2019, three patients with heart disease received iPSC-derived cardiomyocytes. While some researchers are patiently waiting for the results of these clinical studies, others are pursuing new therapeutic strategies. For example, stem cell biologists are attempting to repair mutated genes in human iPSCs using CRISPR-Cas9 technologies. In theory, these genetically modified cell therapeutics could then be administered to patients in need.
Finally, there has been tremendous interest among researchers to leverage iPSC technologies for human disease modeling. In particular, the focus has been on the modeling neurologic diseases, such as Alzheimer’s disease, Parkinson’s disease and dementia.
What will the future hold for iPSC-derived cell therapeutics? Unfortunately, we’ll have to await the clinical data to find out.
To learn more about the market for iPSCs, view the “Global Induced Pluripotent Stem Cell (iPS Cell) Industry Report, 2021.”
What questions do you have about iPSC clinical trials? Ask them in the comments below.