GUEST POST: This is a guest post by Anthony Stonefield, Co-founder & Executive Chairman of AutoRegenic Cell Laboratories (New York, New York). AutoRegenic Laboratories has developed an unique set of laboratory techniques, procedures and materials for producing patient-specific stem cells through nuclear transfer that is showing encouraging potential to become a practical, ethical and affordable approach for conducting spinal cord tissue re-origination.
He is a serial entrepreneur in innovations with 24 years of experience in venture development globally.
The Emergence of Miracle Cell Medicine
It is within this challenging context that I found an independent team of applied researchers who appeared to have truly gate-crashed the future of personalized regenerative cell therapy.
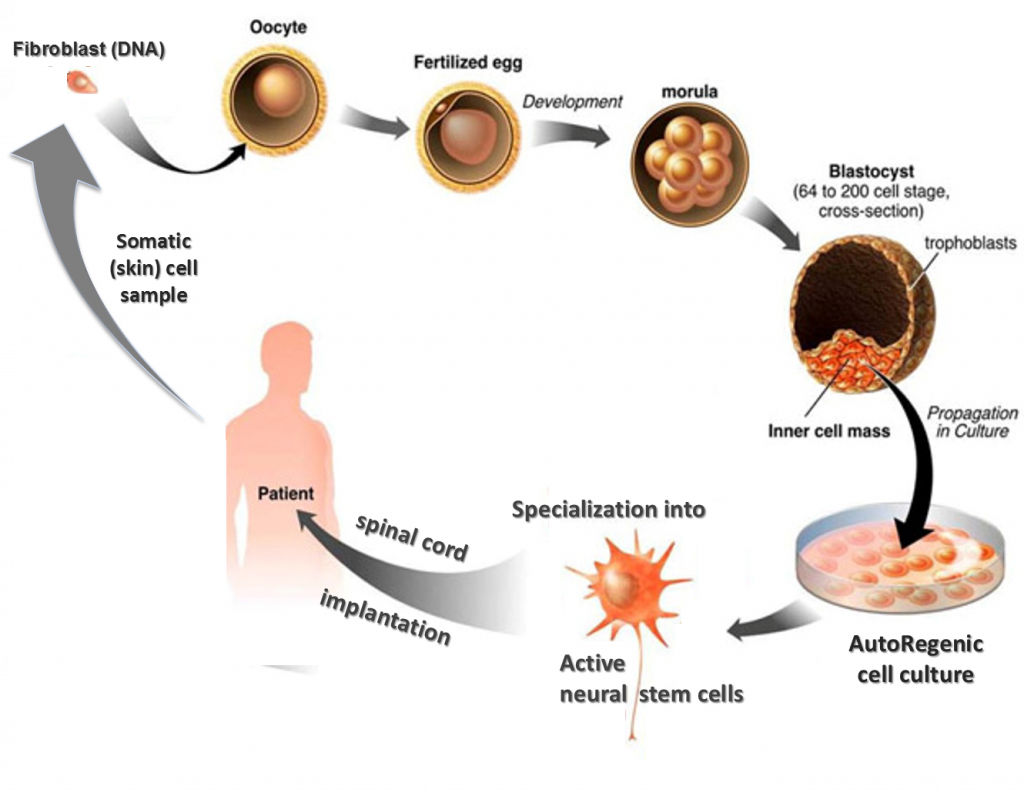
Using a modification of SCNT—a process that commandeers the fertilization process to “naturally” render stem cells—the team can reliably produce vigorous and effective naïve pluripotent stem cells in an ethically sound manner that is both practical and affordable enough to put personalized endogenous biomedical treatments in reach of ordinary people. The cells are re-birthed from your own DNA (from a skin sample), ready to be multiplied and specialized to regrow your tissues.
The team refers to the product as autoregenic cells (ARC) and have chosen to operate largely in stealth mode, beholden only to science and patients.
ARC cells actually work. Over the last 10 years, the ARC team conducted dozens of positive in-vitro experiments with animal and human tissue and several in-vivo experiments with animals. Then in November 2012, the team supplied neural ARCs for a pioneering treatment of a complete quadriplegic man, Tommie Prins, who has made striking progress from his complete paralysis and numbness – see www.facebook.com/MakingTommieWalk.
This experimental treatment proved that human spinal cord tissue did regrow, the implanted ARCs did not precipitate tumors or secondary ailments, and the patient’s condition improved measurably and lastingly. On that basis, the government of South Africa approved a clinical trial to treat more quadriplegic patients with ARCs.
Medical research review and regulations are fairly robust there. The first trial subject is expected to be treated later this month.
Autoregenic Cells (ARC) for Treatment of Quadriplegia
Severe spinal cord injury disrupts the single “data cable” that connects the brain to the body. Historically nerve damage has been irreversible, with the notion of repairing nerves bordering on impossibility. Quadriplegia has, until now, represented a life sentence of numbness, paralysis and despair for its victims and their families.
The film “Me Before You” and the life of Superman actor Christopher Reeve offer insight into the plight of victims. Suffice it to say that an ARC treatment for the world’s over a million victims of severe spinal cord injury (and more than 25,000 new victims each year) represents dizzying promise in terms of soothing suffering, restoring dignity and reducing the cost of care.
To realize this promise, the ARC team has been expanded to include business and advisory members, including me. We are completely focused on productizing the innovation as quickly and safely as possible. While the team’s laboratory processes are trade secrets, we have engagements with a selection of researchers, universities and clinics wherein a range of autologous applications are being explored.
We are working to refine the ARC manufacturing and implantation protocols and to define a commercial model for bringing the innovation to as many patients as possible, as quickly and safely as possible.
In the spirit of democratized healthcare and to sustain our current pace and independence, we’ve launched a crowd funding campaign.
I hope you will help our cause by donating at http://www.medstartr.com/project/detail/772-Expediting-a-Promising-Stem-Cell-Treatment-for-Quadriplegia.