Of course, I am always interested in technologies transferred from Georgetown, because I attended the university for Graduate Studies in Biochemistry and Molecular Biology.
Headquartered in Rockville, Maryland, Progagenix was founded in 2014 by Brian Pollok, Ph.D. Pollok is the company’s Co-Founder, as well as its current President and CEO. I am honored to release this interview in which we discuss the company’s core technology, its preclinical-stage programs, and future goals. Enjoy.
Interview with the Brian Pollok, Ph.D., Propagenix CEO
Cade Hildreth: What is your professional background?
Brian Pollok: I received my Doctorate in Molecular & Cellular Biology and completed a Damon Runyon Cancer Fund Post-doc in Molecular Immunology. I have also been Faculty at Wake Forest University, a Scientist at Pfizer, and held R&D leadership positions at Aurora Biosciences/Vertex, as well as Invitrogen/Life Technologies as CSO.
I was also the past President of ATCC and have been a biotech entrepreneur since 2012, beginning as the University of Virginia’s first Entrepreneur-In-Residence.
Cade Hildreth: How did you become involved with Propagenix?
Brian Pollok: I attended a research seminar given by Dr. Richard Schlegel of Georgetown University in July 2012 on his discovery of the Conditional Reprogramming (CR) technology. That next week I began working on the start-up, which required engaging with Dr. Schlegel, as well as working with Georgetown University’s Technology Transfer Office on a license agreement, and identifying seed funding.
Cade Hildreth: What year was Propagenix founded and why?
Brian Pollok: Together with Dr. Sherry Challberg, our co-founder and COO, we launched Propagenix in mid-2014. The company rapidly executed the exclusive license with Georgetown and closed on the seed funding in the same week.
The reason for pursuing this start-up was based on two essential properties of the CR technology, which are:
(1) The ability to massively expand primary epithelial progenitor cells from a wide variety of normal and diseased epithelial tissues including solid tumor biopsies,
(2) The ability of the expanded cells to retain their differentiative capacity when removed from the propagation media.
Initially it was thought that the company would focus on validating the performance of this licensed technology for diagnostic applications such as personalized tumor chemotyping.
However, our investors challenged us to think about whether we could also apply CR technology – or a new invention that possessed similar attributes – to approach the potentially much larger cell therapy/tissue engineering market.
Cade Hildreth: I am impressed by the rapid launch of the company. How did Propagenix go about answering this challenge from your investors?
Brian Pollok: Great question. Led by Dr. Chengkang Zhang, our R&D team recognized that while CR technology had powerful properties for culturing epithelial cells, certain features such as the requirement for animal-derived components and irradiated mouse feeder cells could pose significant regulatory barriers.
To address this challenge, the team devised a functional screening paradigm using cocktails of recombinant growth factors and small-molecule signaling modulators that would drive normal epithelial stem cell proliferation without use of genetic elements.
In less than a year, they succeeded in discovering a chemically-defined media formulation that enabled massive propagation of tissue-resident epithelial stem cells while preserving the differentiative capability and the genomic integrity of the expanded cells. We have named this technology “EpiX™” (for Epithelial eXpansion media).
Propagenix has been granted four issued US patents on the EpiX technology, and a publication came out in the October 16th issue of Cell Reports which describes this invention in detail.
Cade Hildreth: This is fascinating. How does Propagenix deploy these two different platform technologies, CR and EpiX?
Brian Pollok: The EpiX technology is geared to bioproduction of tissue-resident epithelial stem cells, not only for cell therapy applications but also for creating superior functional diagnostic assays and for research use in drug discovery and consumer product testing.
In contrast, CR technology is primarily used by us to generate patient-specific tumor cell cultures for oncology research, but it has also been deployed within the academic research community for propagation of many sources of primary epithelial stem cells including airway, ocular, cochlear (ear) and GI tract tissues.
Cade Hildreth: Has Propagenix technology been validated by external partners?
Brian Pollok: I am happy to share that there have been over 30 peer-reviewed publications using the CR technology. We have worked with a number of biopharma oncology groups to create patient-specific CR tumor cultures, most notably with AstraZeneca which led to a co-publication in Molecular Cancer.
EpiX technology has been extensively validated by multiple academic labs as well as CROs, biopharma, and consumer product companies for uses ranging from creating tissue models for xenobiotic testing all the way to large-scale cell bioproduction for organ transplantation.
Cade Hildreth: That is a great deal of third-party validation. What additional technologies is Propagenix currently developing?
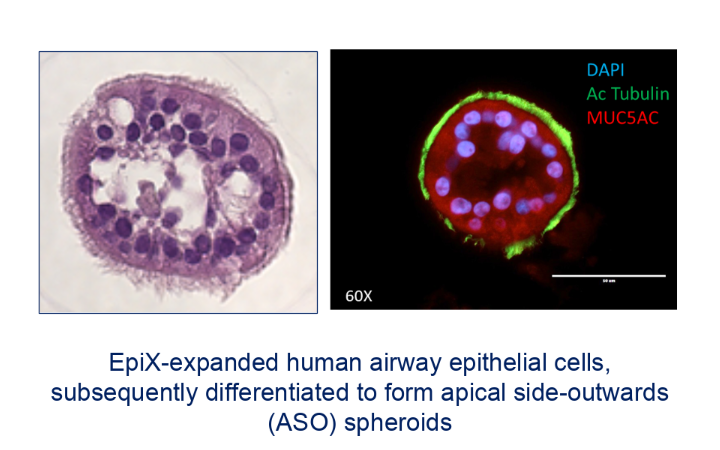
Brian Pollok: This is an important question. We are focusing significant attention on how to best organize expanded epithelial stem cells into mature tissue structures. For example, we have developed methods for the efficient expansion of airway epithelial cells from very small nasal brushing samples and for the downstream differentiation of such expanded airway cells in submerged conditions, in both planar and apical side-outward organoid configurations.
These methods are enabling for a variety of applications. One program funded by an NIH SBIR grant is to create a personalized “theratyping” companion diagnostic assay for drugs that act directly on airway cells such as the current CFTR modulators for cystic fibrosis. Another grant (in collaboration with Yale University) is directed at creating re-cellularized tracheal scaffolds using Propagenix technology.
Also, our work on airway organoids is generating considerable interest due to unique attributes that are of significant value in drug discovery and development programs in respiratory diseases ranging from COPD to infectious disease vaccine development.
Cade Hildreth: Are there preclinical-stage programs that the company is advancing?
Brian Pollok: Because of the long proliferative runway provided by the EpiX technology, it enables the ex vivo genetic engineering of these primary cells. Using this capability, we are advancing an autologous tissue therapy for one form of Epidermolysis Bullosa (EB), a set of skin blistering diseases for which there is only marginal palliative treatment.
We are paying particular attention to insure that a vital quota of gene-repaired keratinocyte stem cells are present in the engineered skin graft and that a robust basement membrane structure forms. These features are crucial to creating a durable therapy for these patients.
Cade Hildreth: What differentiates Propagenix from other cell therapy companies?
Brian Pollok: Our sole focus in on the use of tissue-resident epithelial stem cells for biomedicine. We do not work in the pluripotent stem cell area, nor in the mesenchymal stem cell field. Our platforms can deliver clinical-scale quantities of primary epithelial cells, which are readily differentiated into functional engineered tissue structures.
Using a sports analogy to compare our technology with iPSC technology: Why would you run a marathon when you need only run a 5K race to reach the finish line?
Cade Hildreth: What do you see as Propagenix’s three- to five-year goals?
Brian Pollok: We have three clear goals to complete during this time, which are:
- Timely completion of our preclinical work on the engineered skin therapeutic for EB.
- Establishing mutually beneficial partnerships in therapeutic areas using our cell bioproduction technology.
- Execute on a balanced business model that leverages our technology to generate non-dilutive revenue and complements our capital funding.
Cade Hildreth: How did the Maryland Stem Cell Research Fund help Propagenix and how is such an organization important to the field?
Brian Pollok: I’m glad you asked, because the Maryland Stem Cell Research Fund has been instrumental in providing both financial support and visibility at critical times. The Commercialization Grant that we received in 2017 helped drive application data that aided us landing a significant license with StemCell Technologies and also our successful application for SBIR funding from NIH.
Having a champion at the state level which takes an expansive view of how to assist the stem cell industry in Maryland has been a boon for our company growth.
Cade Hildreth: Final and most important question! How can people get it touch with you or Propagenix to learn more?
Brian Pollok: Folks can contact us through our website (www.propagenix.com) or calling the support line at 240-713-3300. We would love to hear from like-minded partners and individuals.
Do you have additional questions about Propagenix and its unique technology platform? Ask them in the comments below.