Authored By: Aristotle Kayafas, Trailhead Biosystems Inc.
60,000 miles of blood vessels run all throughout the average human body (Cleveland Clinic, 2022). Mostly tiny arteries and veins, they carry life-giving blood to every part of the body, enough to make the 5800-mile round trip between New York and San Francisco close to ten times.
Every millimeter of those sixty thousand miles is covered in a layer of specialized endothelial cells, forming one of the largest organ systems in the human body (Cleveland Clinic, 2022). These cells line every blood vessel, and therefore are critically important in cardiovascular health. The endothelial cells come in direct contact with blood, serving as a barrier as it flows through blood vessels. Highly specialized, endothelial cells have important roles in sensing the blood’s osmolarity and oxygen content through specialized molecular sensors, and they intricately communicate to other cells in the blood vessels to dilate or contract (Cleveland Clinic, 2022) (Alberts, et al., 2002). This precise control over contraction, known as vascular tone, plays a major role in regulating blood pressure.
Endothelial cells also play a big role in the remodeling and repair of blood vessels following injury. Endothelial cells respond to signals in the surrounding tissue such as those induced by hypoxia, and they proliferate towards the origin of the signal, VEGF, in a process known as angiogenesis (Alberts, et al., 2002). Understanding how endothelial cells respond to these angiogenesis signals is critical to understanding the healthy function of the cardiovascular system.
Unsurprisingly, endothelial cells are also some of the first specialized cells to develop. In the developing embryo, they form the first elements of the vascular system. The heart descends from uniquely differentiated endothelial cells. This nascent organ forms from the looping of endothelium which creates the four-chambered heart. When the heart begins to beat, the blood vessels have been built around it, and the vascular system is fully integrated from its birth.
Hematopoietic stem cells, those that give rise to blood inside the vessels, also originate from uniquely specialized hemogenic endothelial cells (i.e. capable of forming blood). Veins, arteries, and the blood inside them all share a similar origin.
Endothelial cells form nascent blood vessels. Additional layers (such as pericytes and smooth muscle support cells) are added to the endothelial cells as needed to properly form more mature blood vessels (Alberts, et al., 2002). The smallest vessels in the body, called capillaries, however, are constructed solely of endothelial cells and basal membrane even in adulthood (Alberts, et al., 2002). As we grow and age, endothelial cells are critical to the normal, healthy development and operation of the entire cardiovascular system and the function of blood. As such, when these cells malfunction, serious complications can arise.
Malfunctions in endothelial cells manifest primarily through cardiovascular maladies. One example of endothelial cell malfunction is atherosclerosis. In atherosclerosis, the endothelial cell lining of blood vessels becomes damaged, allowing for the formation of plaques as fatty residues inside blood accumulate at the damage site (Ashorobi, Ameer, & Fernandez, 2023). These plaques can build up and completely block the vessel, restricting the flow of blood and increasing blood pressure.
The clots can also break off through thrombosis, which is particularly dangerous to cardiovascular health. Thromboses can travel within the circulatory system until they reach a small-diameter blood vessel and block blood flow to the surrounding tissues. These blockages can cause strokes and heart attacks, meaning that thromboses and their resultant maladies are the most common cause of death in developed countries (World Health Organization, 2023).
Heart disease is the leading cause of death in the United States and around the world, according to the CDC and WHO (World Health Organization, 2023). 850,000 people in the United States suffer a heart attack every year, per the CDC. Nearly 1 in 5 people over the age of 65 report having some form of heart disease (Centers for Disease Control and Prevention, 2023). 695,000 Americans died of cardiovascular disease in 2021 (Centers for Disease Control and Prevention, 2023). In other words, 1 in 5 Americans that died in 2021 died of heart disease (Centers for Disease Control and Prevention, 2023). 17.9 million people around the world die every year from heart disease, according to the WHO. In the United States alone, these diseases cost an estimated $240 billion between 2018 and 2019 (Centers for Disease Control and Prevention, 2023). The role of endothelial cells in disease goes beyond strictly the cardiovascular, however.
Endothelial cells, through their role in regulating blood flow and in the remodeling of blood vessels, additionally play an important role in the formation of cancer. Just as normal healthy cells require blood supply, cancer cells do too. Furthermore, those cancer cells can over-express signaling factors that drive angiogenesis (Liang & Liu, 2021) (Alberts, et al., 2002). Co-opted by the tumor, endothelial cells respond to angiogenic signals, and as a result vascularize the tumor, essentially hijacking blood supply from healthy tissue. Currently, researchers are investigating how these erroneous signals from the tumor can be disrupted to cut off blood supply to tumors.
Currently, treatments for endothelial cell dysfunction include medications, like aspirin and statins to address clotting and cholesterol. These therapies have been used for many decades, but have their limitations. Statins, for example, can produce muscle pain and increased strain on the liver (Mayo Clinic Staff, May) and their use does not address the mechanism of the formation of the plaque; only the level of hepatic produced cholesterol. These drugs only address the symptoms of endothelial cell dysfunction – they do not address the dysfunction itself. Cell-based therapies for endothelial-related diseases are in development, but they have yet to meet demand at a level that allows those therapies to be developed quickly and used at a large scale (Liang & Liu, 2021).
Investigations of endothelial cell biology requires the availability of cultured cells that faithfully represent the normal biology of the endothelial cell type. Today, a particularly commonly used tool is the HUVEC type of endothelial cultures. These human umbilical vein endothelial cells can be obtained from discarded umbilical cords, but they suffer from a lack of representative biology of the endothelial cells. They are typically grown for multiple passages, which causes loss of specialized functions. It is not a consistently produced cell material, and the product varies, dependent on donor and the user’s prior culturing. One major challenge to develop endothelial clinical therapy is the difficulty in culturing endothelial cells with high efficiency. While other primary endothelial cell types besides HUVECs exist, they also suffer from the same problems associated with HUVECs. At present, the promise of stem cell-derived endothelial cells is high, but challenges in the reproducible and scalable production of e.g. iPSC derived endothelial cells have troubled the field.
Trailhead Biosystems seeks to unlock the life-saving potential of deploying these therapies to more patients by applying their novel technology of HD-DOE. The company has developed a platform that addresses these limitations, and it seeks to build multiple specialized cell types and offer these as products to enable investigations of human disease. High Dimensional Design of Experiments (HD-DoE) is used to develop the knowledge and expertise needed to grow cells at large scale, and with reduced cost. Typically used in engineering contexts, DoE allows researchers engineers to predict measure the effect of multiple variables as opposed to the traditional one factor at a time (OFAT) method of scientific experimentation. HD-DoE goes beyond the normal DoE to help understand to explore a greater number of dimensions.
The benefit of high dimensional testing is that it allows for a thorough, detailed interrogation of the experiment across many variables. The high dimensionality afforded by HD-DoE allows for extremely efficient experimentation. Consequently, the quality of Trailhead’s HD-DoE experiments instills a high degree of confidence in the robustness of their protocols. Within the past year, Trailhead’s biological engineers used HD-DoE to develop a protocol to induce the controlled differentiation and production of endothelial cells from iPSCs.
Trailhead’s endothelial cells are of high quality, purity and are offered at a substantially more reasonable price point than has been brought to market thus far.
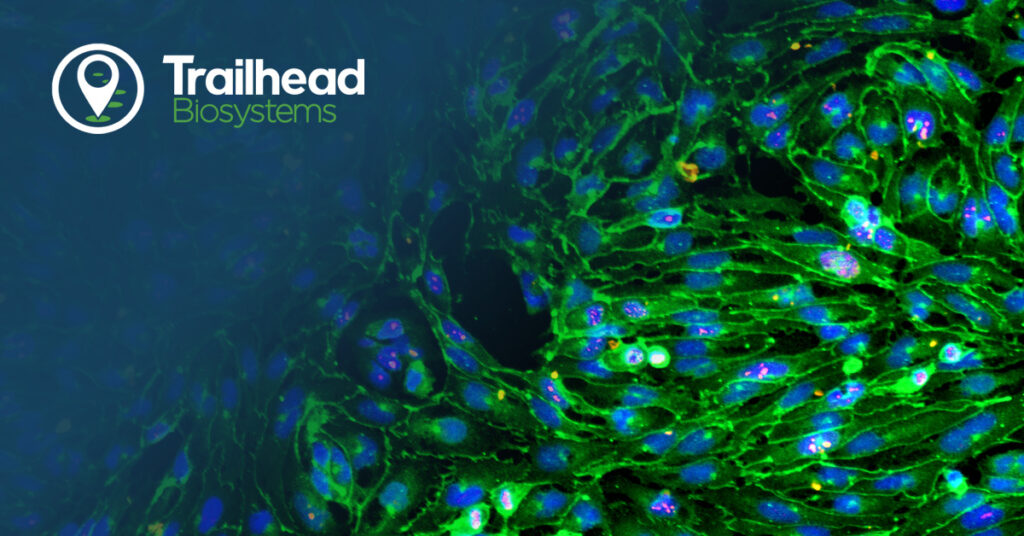
Functional analysis demonstrated the ability of these new iPSC-derived endothelial cells to express the scavenger receptor for acetylated low-density lipoprotein (LDL), a critical function of endothelial cells in the human body. Furthermore, these cells demonstrated in vitro tube formation, characteristic of angiogenesis. Nitric oxide, important in the signaling function of endothelial cells, was also produced by Trailhead’s iPSC-derived endothelial cells.
The molecular signature of the product adheres to the known profile of early arterial/venous mixed phenotype endothelial cells. RNA sequencing data shows expression of endothelial ETS transcription factors, ETS1, ERG, and FLI1. Expression of venous marker genes, such as NR2F2 and EPHB4, and arterial marker genes, GJA4 and NRP1, are observed at a high level. Furthermore, they express the tight junction proteins TJP1 and PECAM1, while also expressing SOX7, SOX17, and SOX18 transcription factors. Importantly, the endothelial cells express tyrosine kinase receptors for VEGF signals like KDR and FLT1, and low expression of stemness genes, further confirming the differentiation into endothelial cells from iPSCs. These RNA sequencing results were reinforced through numerous immunofluorescence assays and qPCR targeted to those genes.
These cells will be of great use to any researcher that studies iPSC-derived endothelial cells in the context of drug discovery, angiogenesis, and human disease modeling as examples. By offering the cells in large quantities, 3D printing of vascular beds necessary for organ/tissue building is also facilitated. Gene editing and modeling of congenital vascular disease may also be envisioned. The study of angiogenesis using Trailhead’s endothelial cells is a perfect example. A researcher would have a large, pure population with which to test the effects of different compounds on angiogenesis. Trailheads iPSC-derived endothelial cells also could be used to build in vitro disease models. The lowered cost of Trailhead’s endothelial cells will unlock new, potentially untapped pathways for investigation as experiments become economically viable. The cost-efficiency of Trailhead’s endothelial cells has the potential to not only accelerate existing research but may also unlock new areas of research.
Trailhead’s iPSC-derived endothelial cells are now officially available for public purchase. Offering both consistency and affordability, these cells promise to be a transformative resource for researchers. Those eager to push the boundaries of endothelial cell research can purchase them now at shop.trailbio.com.