Biotech founder and CEO Jan Jensen has never been one for following the beaten track. As a young scientist studying the formation of insulin-producing pancreatic cells, Jensen questioned the prevailing view that these cells descended from neuroectoderm. His pancreatic embryonic tissue studies told him otherwise: insulin-producing pancreatic cells originate from within the pancreas itself. Jensen’s hypothesis, now the accepted view, and his further studies made him the first developmental biologist at Novo Nordisk, at a time when the world’s largest insulin producer had little interest in cell-based therapy. Now, 20 years later, Novo Nordisk is at the forefront of cell-based Type I Diabetes research.
In 2007, Cleveland Clinic recruited Jan to apply his world-class expertise to two novel areas: regenerative medicine, and cell therapy for diabetes. The Cleveland Clinic continues to be ranked among the finest health care systems in the world, and Jan found himself surrounded by preeminent physicians, wishing to make an impact as a developmental biologist upon patient care.
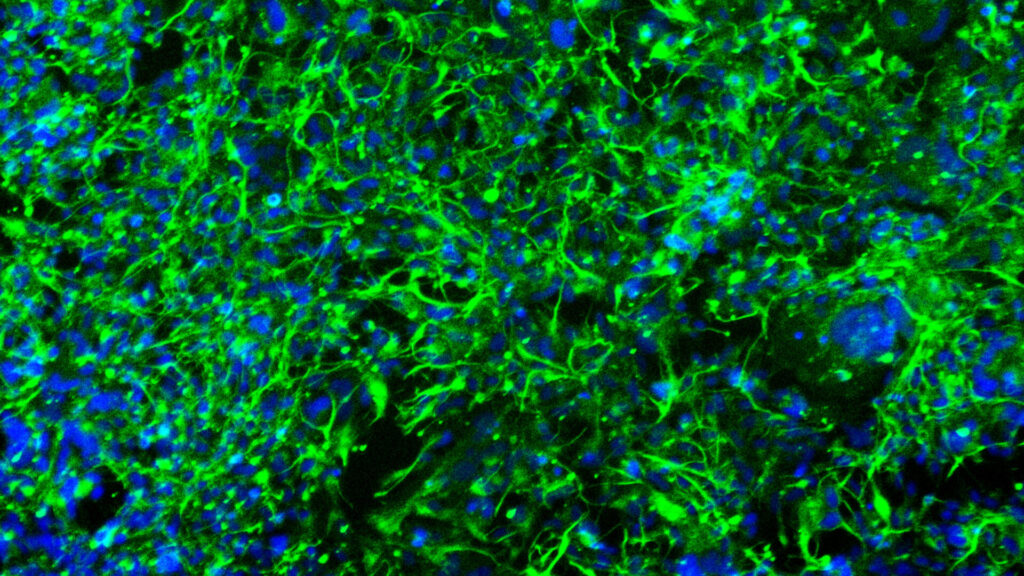
Jensen then found himself faced with the opportunity to solve the problem of connecting lab knowledge to the exam room and operating table. In response, Jan founded Trailhead Biosystems to shift paradigms in both drug development and cell-based therapies. In addition to recently moving in to their new 12,000 sq ft facility, Trailhead recently launched their first remarkable product: iPSC-derived Oligodendrocytes.
This novel product will provide a powerful, unique human cell-based model which has the potential to become a drug development game-changer for the study of demyelinating diseases such as Multiple Sclerosis and Leukodystrophies.
“We are constantly in awe of the powers of the HD-DoETM technology. This platform empowers us to uncover and harness the secrets of human development to create better specialized cell products in a very efficient manner,” says Katie Sears, of Trailhead’s Bioinformatics team.
On the heels of recent FDA regulatory guidelines which allow biotech and pharma companies to limit, or in some cases even forgo, the use of animals, Trailhead’s promise of generating specialized human cells by the billions is timely and welcomed for many researchers. Case in point: Trailhead’s iPSC-derived OPC protocol drastically reduced the standard timeline of differentiation from iPSC to OPC from as many as 90 days to just around 20 days, with billions of cells generated per batch.
As Jensen puts it, “Trailhead Biosystems has a vast opportunity to deliver on the yet unfulfilled promise of iPSCs. Currently, there are transformative events occurring in the space of AI-driven drug discovery, but at the end of the day, each AI-predicted drug needs to be validated. Improved iPSC-derived cells not only help in such validation, but also in drug toxicity and safety assessment. Furthermore, they empower entirely new and better drug screens using compound libraries.”
Today, pharmaceutical companies live with enormous early-lead attrition. By shifting towards a human cell-based system, with relevant cellular physiology and behavior, researchers can now avoid disappointing or lackluster results, and the astronomical expenses that come along with animal-based, translational studies. They will, in turn, increasingly enjoy a reduction in development time and cost, and ultimately will also have reduced risk of clinical failure. Consequently, studies will be faster and more reliable, with less guesswork.
How does Trailhead deliver these cells at a fraction of the time and with such quality and consistency? This efficiency is entirely based on the power of machine-enabled combinatorial testing. Starting with a question, Trailhead’s R&D teams kick their math skills and developmental biology expertise into gear and employ their unique HD-DoETM platform (with the assistance of custom robotics) to determine better and more efficient ways to drive an iPSC toward its final identity from thousands upon thousands of conditions.
According to Nooshin Amini, head of the ectoderm team, “Trailhead Biosystems’ mathematically driven experiments are vastly superior to human hypotheses. Cells never rely on a single signaling input –multiple inputs always combine to create the right conditions for it to change. With HD-DoE, we can identify and mimic any cell’s desired microenvironment, and thus we can develop novel differentiation protocols to generate stable, specialized cell types in vitro. We simply emulate the environment that exists in the embryo, but within a bioreactor. The idea is to make only one cell type, and not the hundreds that occur in the embryo, but an iPSC cell only knows how to build an embryo, and therefore we are up against these forces within the bioreactor. To tell a cell to go in one direction usually involves making sure that it does not go the wrong way.”
Trailhead is quickly gaining attention in the stem cell market, attracting researchers who are looking for human-based cells to drive their drug development efforts. In addition to OPCs, they offer other highly sought-after Central Nervous System cell types such as Parvalbumin-positive interneurons, and midbrain A9 subtype dopaminergic neurons among others. Trailhead is not afraid, however, to venture out onto new paths.
Trailhead does not limit itself to brain cells, offering many other cell types of research and clinical interest. Currently, for example, they generate iPSC-derived endothelial cells, hematopoietic stem cells, as well as many other blood lineages. Protocols for pancreatic beta cells and several neuronal and retinal cell types are also in the making. It is likely that Trailhead Biosystems holds the largest number of active programs to create specialized cells from iPSCs, with simultaneous focus on scaled production and delivery. Each of these programs is accompanied by a healthy dose of validation data and RNAseq quality control to satisfy the strict requirements of sophisticated users.
The cells produced by Trailhead have relevance in regenerative medicine, well beyond drug discovery. To enable such clinical development, the company is operating on a partnering model that is molded after the typical risk-stratified pharmaceutical industry model of a novel drug lead (i.e. an API, Active Pharmaceutical Ingredient), but as the products of Trailhead are cells and not drugs, they refer to their clinical candidates as “ACI’s” (i.e. Active Cellular Ingredients) to reflect in future cell-based therapy that the truly active component is the living cell.
It is not difficult to see that Trailhead is not afraid to venture out onto new paths. Their expert utilization of the HD-DoEplatform enables them to quickly pivot and develop novel protocols to generate additional cell types. Moreover, they are open to partnering with researchers who are interested in cell types that are not currently a part of the company’s portfolio.
Trailhead is constantly striving to create a world where cells are integrally embedded in successful therapies for many human diseases. To learn more, head over to their website: https://www.trailbio.com/