PeptiGrowth Inc. (Headquarters: Chiyoda-ku, Tokyo, President: Jiro Sugimoto) has successfully developed a novel synthetic peptide called “Wnt3a alternative peptide (β-catenin pathway agonist)” which has equivalent function to recombinant Wnt3a as well as GSK-3β inhibitors, such as CHIR99021(CHIR)). This product will be available in the middle of November 2023.
Development of Synthetic Peptide Growth Factors by PeptiGrowth
Conventional growth factors and cytokines used in the manufacturing of regenerative medicine and cell therapy products face various quality challenges such as batch-to-batch variation, potential contamination with biological impurities, low stability, and high cost. PeptiGrowth has been working on the development of a series of synthetic peptides that can address these challenges while maintaining equivalent function to the conventional growth factors and cytokines in the market. Our peptides are completely chemically synthesized and animal component-free, enabling Xeno-free and chemically defined cell culture media.
About Wnt3a alternative peptide (β-catenin pathway agonist) [Product code: PG-008]
- The activation mechanism of the β-catenin pathway by Wnt3a alternative peptide
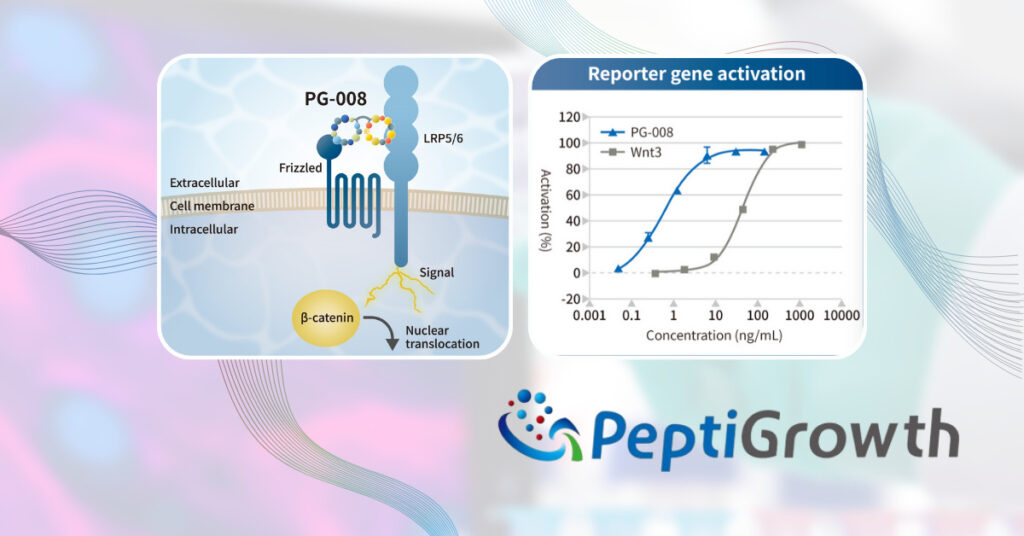
The Wnt3a alternative peptide (PG-008) is composed of two cyclic peptides that have specific and strong affinity to the receptors of Wnt3a, FZD (Frizzled), and LRP (Low-density lipoprotein receptor-related protein) 5/6. Similar to Wnt3a, PG-008 binds to FZD and LRP5/6, activating the β-catenin pathway in the Wnt signaling pathway (see the figure on the right)
- Superior agonist activity compared to recombinant Wnt3a.
We performed a functional comparative test between PG-008 and commercially available recombinant Wnt3a. Specifically, we used the TCF-LEF reporter assay in HEK293 cells, which evaluates the activation of luciferase genes. This assay allowed us to compare the agonist activity of PG-008 and recombinant Wnt3a on the β-catenin pathway. As a result, PG-008 was shown to activate the reporter gene at lower concentrations and exhibit superior agonist activity compared to recombinant Wnt3a (see the figure on the right).
- Achieving cell differentiation at an exceptionally low concentration compared to CHIR99021
Our collaborator conducted an experiment to compare the differentiation efficiency from iPSCs to definitive endoderm (DE) comparing PG-008, CHIR, and recombinant Wnt3a, following the scheme outlined below (Dr Nick Hannan, Univ of Nottingham, unpublished data). It is important to note that CHIR is a small molecule that functions as a GSK-3β inhibitor and commonly used as an indirect activator of the β-catenin pathway in Wnt3a signaling.
When using recombinant Wnt3a (50 ng/mL, approx.1.3 nM), a significant number of undifferentiated cells were observed on Day 4 (the left image below), and the induction efficiency of DE was approx. 60% (the right image below). In contrast, when PG-008 and CHIR were used, a uniform differentiation into DE was observed, and a differentiation efficiency of over 98% was achieved. These results demonstrate that only 1 nM of PG-008 exhibits a comparable differentiation induction efficiency to 3 µM of CHIR. Furthermore, we confirmed that PG-008 does not exhibit cytotoxicity even at a high concentration of 10 µM.
We anticipate that PG-008 will be used for various applications, including induction of differentiation from iPSCs and adult stem cells to endodermal lineage cells and maintenance of various organoids.
- Product Overview
- Product name: Wnt3a alternative peptide (β-catenin pathway agonist)
- Product Code: PG-008
- Product form: Lyophilized
- Storage conditions: -20°C or less
- Purity: ≥95% by HPLC
- Molecular weight: 5099.63(Acetate)
- Size: 10 μg (volume per a glass vial)
- *This product can be provided with an Animal Component Free (ACF) certificate.
- *This product is for testing and research use only (RUO).
- *There is a possibility that the specification would be changed.
- Purchase Our Product
If you would like to purchase our products, please contact us at the information below.
For customers who would like to purchase GMP-grade products or RUO-grade products in bulk scale, please contact us at the Contact Information below.
Development of other peptides from PeptiGrowth
We have developed alternative peptides targeting numerous growth factors and cytokines. To date, we have launched eight products, and we also plan to launch several growth factor alternative peptides targeting PDGF-AA, TPO, FGF2, etc. from the end of 2023 to early 2024. Details will be updated on our website. If you are interested, please contact us at the following Contact Information.