State-of-the-Art Facility Designed to use Clonal Master iPSC Lines as Renewable Cell Source for Manufacture of Off-the-Shelf Product Pipeline
The Company’s cGMP facility, located in San Diego, California, is custom designed for the manufacture of off-the-shelf allogeneic cell products using clonal master induced pluripotent stem cell (iPSC) lines as a starting cell source. The new state-of-the-art facility has been commissioned and qualified, and the Company has been issued a drug manufacturing license by the State of California, Department of Health Services, Food and Drug Branch.
“The on-time launch of our cGMP manufacturing facility is a significant milestone that positions the Company as the leading manufacturer and developer of off-the-shelf NK cell and CAR-T cell cancer immunotherapies,” said Scott Wolchko, President and Chief Executive Officer of Fate Therapeutics. “With full control of cGMP production, combined with our proven ability to genetically-engineer induced pluripotent stem cells and create clonal master iPSC banks qualified for clinical use, we believe we have established operational capabilities unique to the industry to ensure consistent, large-scale, and cost-effective manufacture of best-in-class off-the-shelf cell products for on-demand delivery to patients.”
In early September, Fate Therapeutics announced that the U.S. Food and Drug Administration (FDA) cleared the Company’s Investigational New Drug (IND) application for FT596, its off-the-shelf, iPSC-derived CAR NK cell product candidate engineered to target multiple tumor-associated antigens, for the treatment of B-cell lymphoma and chronic lymphocytic leukemia.
The Company is also conducting first-in-human clinical trials of FT516, an off-the-shelf, iPSC-derived NK cell product candidate engineered to express a novel high-affinity, non-cleavable CD16 (hnCD16) Fc receptor, for the treatment of acute myeloid leukemia and B-cell lymphoma, and FT500, an off-the-shelf, iPSC-derived NK cell product candidate, for the treatment of advanced solid tumors.
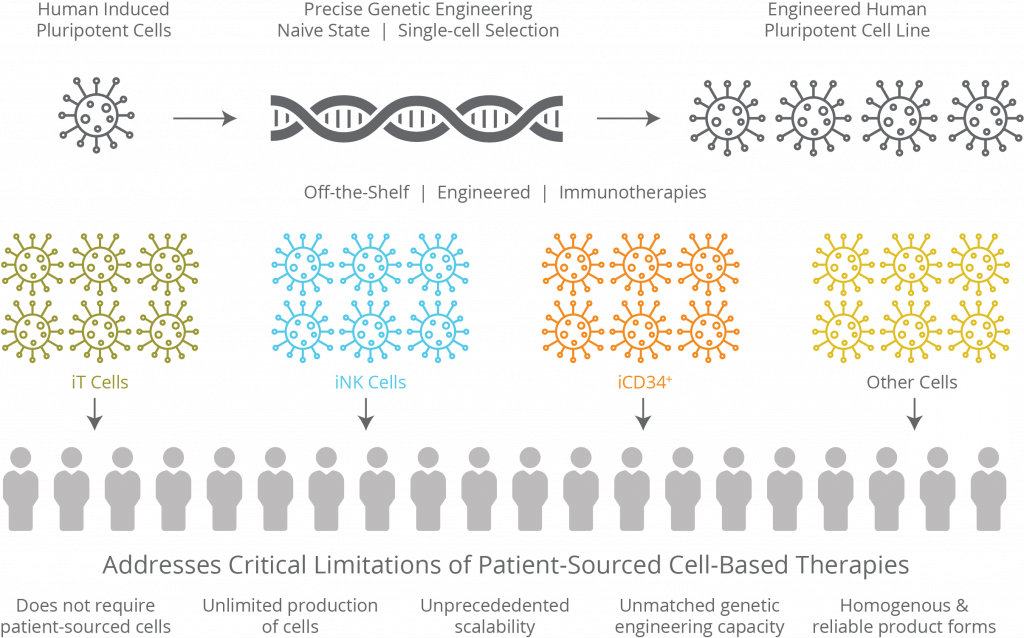
About Fate Therapeutics’ iPSC Product Platform
The Company’s proprietary induced pluripotent stem cell (iPSC) product platform enables mass production of off-the-shelf, engineered, homogeneous cell products that can be administered with multiple doses to deliver more effective pharmacologic activity, including in combination with cycles of other cancer treatments. Human iPSCs possess the unique dual properties of unlimited self-renewal and differentiation potential into all cell types of the body. The Company’s first-of-kind approach involves engineering human iPSCs in a one-time genetic modification event and selecting a single engineered iPSC for maintenance as a clonal master iPSC line. Analogous to master cell lines used to manufacture biopharmaceutical drug products such as monoclonal antibodies, clonal master iPSC lines are a renewable source for manufacturing cell therapy products which are well-defined and uniform in composition, can be mass produced at significant scale in a cost-effective manner, and can be delivered off-the-shelf for patient treatment.
As a result, the Company’s platform is uniquely capable of overcoming numerous limitations associated with the production of cell therapies using patient- or donor-sourced cells, which is logistically complex and expensive and is subject to batch-to-batch and cell-to-cell variability that can affect clinical safety and efficacy. Fate Therapeutics’ iPSC product platform is supported by an intellectual property portfolio of over 250 issued patents and 150 pending patent applications.
About Fate Therapeutics, Inc.
Fate Therapeutics is a clinical-stage biopharmaceutical company dedicated to the development of first-in-class cellular immunotherapies for cancer and immune disorders. The Company has established a leadership position in the clinical development and manufacture of universal, off-the-shelf cell products using its proprietary induced pluripotent stem cell (iPSC) product platform. The Company’s immuno-oncology product candidates include natural killer (NK) cell and T-cell cancer immunotherapies, which are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies, and to target tumor-associated antigens with chimeric antigen receptors (CARs).
The Company’s immuno-regulatory product candidates include ProTmune™, a pharmacologically modulated, donor cell graft that is currently being evaluated in a Phase 2 clinical trial for the prevention of graft-versus-host disease, and a myeloid-derived suppressor cell immunotherapy for promoting immune tolerance in patients with immune disorders. Fate Therapeutics is headquartered in San Diego, CA. For more information, please visit www.fatetherapeutics.com.